Venipuncture in Small Exotic Companion Mammals
(Please click on the PDF icon above to download the PDF version with full illustrations and charts)
Collection of blood for diagnostic testing can be challenging in exotic pet mammals, especially in smaller species. Modern technology allows acquisition of useful information from very small samples, but samples still must be of high quality and meet minimum volume requirements.
Tips for improving sample quality include the following:• Use heparin to reduce fibrin clot formation• Use small needles and syringes to prevent collapse of smaller vessels• Use gentle suction during sample collection to prevent hemolysis of the sample.
Sample volume is limited by patient size. A common guideline is to collect no more than 10% of blood volume. Total blood volumes vary from species to species but can generally be assumed to be 6% to 8% of body weight.1 Therefore, a 100-g hamster has a blood volume of 6 to 8 ml, and maximum volume for collection would be 0.8 ml. Clinical judgment may necessitate adjustments to recommended volume limits.
Analysis of Low-Volume SamplesSamples can be run with in-house equipment or sent to reference laboratories familiar with diagnostic testing in these species. The Abaxis (www.abaxis.com) in-house blood chemistry analyzer can run a chemistry panel on heparinized whole blood samples; practical minimum volume is 0.15 ml of high-quality whole blood. Reference laboratories often can analyze smaller samples but may use dilution techniques. Individual laboratories should be contacted for instructions on submission of low-volume samples. Many laboratories provide smaller serum/plasma gel separator tubes for this purpose.
When sample volume does not allow clinical chemistry or CBC, the CBC can be done manually on a single drop of blood collected in a heparinized hematocrit tube. Information that can be obtained from a manual in-house CBC includes hematocrit, differential white blood cell count and analysis of the morphologic characteristics of both red and white blood cells, complete blood count using a hemocytometer, and measurement of total plasma solids (Figure 1). To prevent artifacts, keep in mind that blood smears should be made immediately after collection when heparinized samples are being used.
Sample collection in small exotic mammals takes practice. The collection site depends on the species, the condition of the patient, and the practitioner's preference. The following guidelines are offered based on the author's personal preference and experience. Others may advocate techniques or sites not mentioned in this article. The "correct" procedure is that which results in consistently good results with optimal patient safety.
Blood Sample Collection SitesThe choice of sample site is based on clinical experience and practitioner preference. My preferences are listed in Table 2. The greatest limiting factors in regard to site selection are patient size and whether the patient can be safely restrained.
Risks of Blood Sample CollectionVenipuncture inherently involves some degree of risk, including risk of injury or death caused by restraint and handling. Risks of blood collection itself include vessel laceration and hemorrhage, infection, inadvertent damage to adjacent soft tissue structures, and hypotension due to suddenly decreased blood volume. Careful patient evaluation, restraint, and technique help minimize risk, and experienced exotic practitioners do not report higher complication rates from venipuncture than traditional pet practitioners.
RestraintRestraint is relatively easy in larger species, such as ferrets and rabbits, but becomes increasingly challenging in smaller species. The method of restraint often depends on the preferred collection site. In some cases, sedation or anesthesia is appropriate and necessary, especially when considering collection from the vena cava in species other than ferrets.
In my experience, most ferrets can be safely restrained manually for blood collection by scruffing and stretching, or by rolling the patient in a towel with the head, neck, and forelimbs exposed (Figure 2). However, some ferrets will not tolerate this form of restraint.
Rabbits are prone to self-injury, especially luxation and subluxation of the vertebrae, during restraint and handling caused by kicking with their powerful rear legs. Restraint is used to minimize this risk.2 I prefer using a "burrito-style" towel wrap with either the head or caudal portion of the rabbit exposed, depending on the desired collection site (Figure 3).
Guinea pigs and larger rodents can often be restrained by simply grasping them securely around the shoulders and thorax (Figure 4) or with a towel wrap. Manual restraint of species smaller than guinea pigs for blood collection is extremely difficult.
Sedation/AnesthesiaFor individuals where manual restraint for sample collection is difficult and risky or patient struggling is excessive, sedation and/or anesthesia should be considered. The risk of sedation and anesthesia must be balanced against the risk of foregoing diagnostic blood testing. A number of sedative and anesthetic protocols can be considered, and the method chosen should be determined according to a number of factors, including the patient's overall condition. Methods include simple sedation and anesthesia with injectable and/or inhalant agents (Table 1).
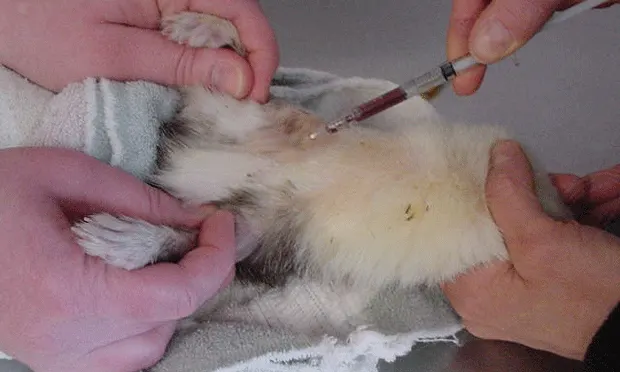
Sample Collection from the Vena CavaExotic practitioners have used the vena cava as a safe and effective site for sample collection for many years.3 Thorough understanding of the anatomic relationship of the vessel, the heart, and external landmarks greatly reduces risk. In ferrets, the vena cava is exceptionally long due to the relative caudal placement of the heart in the thoracic cavity. The vena cava is surrounded and protected by fat and is accessible just below the notch of the sternal manubrium. Correct needle placement is very shallow and poses no risk for inadvertent penetration of the heart (Figure 5).
The cava is shorter in other species, and the distance between accessible vessel and the heart decreases progressively along with patient size. Use of small, short needles (1/2 and 5/8th inch) reduces risk; however, in all but the smallest guinea pigs, these needles are too short to reach the heart. In other smaller species, risk for cardiac penetration is greater, and the practitioner must avoid advancing the needle into the thoracic cavity. It should be noted that in most cases, inadvertent cardiac puncture is not associated with severe complications or death.
Vena Cava PunctureThe general procedure for vena cava puncture is as follows:• Isolate the center of the cranial sternum and manubrium.• Insert a 25- to 27-gauge needle to the right or left of the manubrium at a slight angle aiming for the opposing hip.• Advance the needle while applying negative pressure, redirecting slightly until a flash of blood is detected. The vessel is very close to the surface under the cranial sternum, and it is not necessary to advance the needle very far.• Blood should flow readily after detecting the flash. Failure to flow often means the needle was advanced into and through the vessel, or the bevel was obstructed by the vessel wall. Pull out, rotate, and/or redirect slightly until blood flow improves.