Maximizing Diagnostic Utility of Urine Sediment Evaluation
Elaine Anthony, MA, CVT, St. Petersburg College, Largo, Florida
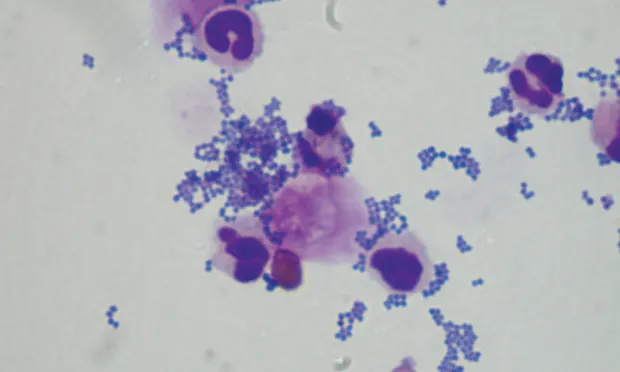
The primary purpose of microscopic examination of urine sediment is to detect abnormal formed elements (eg, cells, casts, crystals) in the sample.
Urine sediment may contain a variety of miscellaneous components, some that have clinical significance (eg, parasites, bacteria), and some that are strictly contaminants (eg, starch granules, pollen, grass particles). The presence or absence of specific formed elements can provide detailed diagnostic information.
Urine sediment examination should utilize 5 mL of fresh urine.1 Normal reference ranges are based on a particular urine amount and any alteration of the amount should be considered. However, it is more important that technicians are consistent when obtaining and evaluating the urine than the amount of urine obtained. Every practice should clearly communicate its protocol and standards to ensure accuracy.
The urine sample should be centrifuged at a low speed (eg, 1500–2000 RPM) for 5 minutes. The supernatant is then decanted and the sediment resuspended in the remaining urine. A drop of this suspension is placed on a glass slide (Figure 1) with a cover slip and examined microscopically.

Placing a drop of suspension on a glass slide. Photo courtesy Vivian Tiffany, BA, CVT.
Unstained Urine Sediment
To evaluate the unstained urine sediment slide, first scan the slide with a low-power objective lens (10×) and subdued lighting (iris diaphragm partially closed and condenser in the lowered position).1,2 To ensure the viewing level is actually urine sediment and not the cover slip or slide, find and focus on any cellular component.2 Once this is established, perform a scan on low power. Large formed elements (eg, casts, crystals) or clumps of cells are evident at 10× magnification. Overall cellularity can be assessed, and bacteria, parasites, and other infectious agents can be seen. Next, proceed to the high-power (40×) objective lens while slightly increasing the light and raising the condenser.
Stained Urine Sediment
Supravital Sediment
A wet preparation may be desired as a follow-up to enhance the refractive index for examination (see Wet- and Dry-Preparation Stains). One advantage of the supravital stain is that it does not contain a fixative, which dissolves crystals. Counts must be performed on the unstained sediment slide first, as quantifying the sediment’s elements once diluted with the stain yields inaccurate results.
Problems may result from a supravital stain that can actually make the identification more complicated; for example, if bacteria and stain precipitant accumulate over time and are not filtered, they can be unintentionally added to the urine sediment. In addition, the stain will occasionally stain cytoplasm the same color as the nucleus, making it difficult to clearly differentiate them. To see nuclear and cytoplasmic detail, particularly when identifying nuclear criteria of malignancy, oil immersion is needed, which the supravital wet preparation does not allow.
Romanowsky Sediment
When cellular elements are difficult to identify, the preferred (and simple) method is to make a dry-preparation or air-dried cytology slide (see Wet- and Dry-Preparation Stains). The slide is stained with a Romanowsky-type stain, such as Diff-Quik. There is comfort in viewing an air-dried cytology slide on oil immersion because of its correlation to peripheral blood—red and white blood cells are easily identified. From this vantage point, other elements can be compared with cells that are now easily identified (eg, blood cells vs transitional epithelial cells). With wet-preparation evaluation, white blood cells become more difficult to identify when swollen, and bacteria can be challenging to detect when found in groups or when contaminants are involved. The dry-preparation cytology stain procedure can help differentiate these aspects and confirm what was seen on direct sediment evaluation. If a cluster of suspicious transitional epithelial cells is encountered, further evaluation under oil immersion is needed for nuclear criteria of malignancy.2
Wet- and Dry-Preparation Stains
Cells can be difficult to identify or differentiate using unstained urine sediment analysis because swollen white blood cells may be confused with renal epithelial cells, or contaminant material may be confused with formed elements. Clusters of transitional cells require further examination on oil immersion to determine if they are normal, reactive, or neoplastic via evaluation of the stained urine sediment:
Wet-preparation supravital stain
Sedi-Stain; new methylene blue
Mix a drop of stain with the suspended sediment and incubate at room temperature for 2–3 minutes before placing a drop of stained sediment onto a microscope slide.
Dry-preparation Romanowsky stain
Diff-Quik (Figure 2)
Wright-Giemsa stain (Figure 3)
Place one drop of suspended sediment on a clean microscope slide. Using a second clean microscope slide, make a compression smear (Figure 4). Air dry the top slide thoroughly and stain gently.

Figure 2
Diff-Quik slide preparation materials
Tip
Because of the low-protein nature of urine specimens, cellular components of urine sediment may not adhere to the slide.1 Agitation in the staining process can also result in loss of the sediment from the slide. Increasing the fixative time will improve the cell’s adherence; more than 1–2 minutes of fixative time for urine cytology is recommended.3 Stain as gently as possible, considering the rinse of a gentle flow of water. Staining time may be 1.5–2 times the amount used for hematology slides, depending on the cellularity of the sediment. The slide should then be air dried thoroughly and evaluated on low-, high-, and oil-immersion.
Conclusion
Microscopic examination of urine sediment is a rapid, easy-to-perform diagnostic procedure that provides valuable information. To be of greatest value, efforts must be made to standardize the performance and reporting of results. Unstained and air-dried cytology can enhance the amount of information for reliable results.
Editor’s note: This article was originally published in April 2014 in Veterinary Team Brief as “Urine Sediment Evaluation.”