Imaging of the urinary bladder in dogs and cats is typically performed to help identify a cause for general lower urinary tract disorders.
Limitations of Radiography
Evaluation of the urinary bladder via radiography is limited because soft tissue and fluid have the same opacity; however, radiographs can help identify mineralized struvite or calcium oxalate urinary calculi.1 Digital radiography has increased resolution and the ability to detect other types of calculi (including cystine and urate calculi in a model and 2 cases),2 but double-contrast cystography via catheterization and administration of iodinated contrast medium, as well as carbon dioxide or room air, into the urinary bladder should also be used to optimize the chance of seeing calculi via radiography.1,3,4 Most urinary bladder tumors do not mineralize and cannot be identified on survey radiographs.
Ultrasonography Guidelines
Ultrasonography is a noninvasive imaging method that can evaluate for soft tissue masses, urinary calculi (regardless of stone composition), cystitis, and ectopic ureters and provide guidance for obtaining samples via percutaneous aspiration or traumatic catheterization (see Traumatic Catheterization).
Traumatic Catheterization
Traumatic catheterization can be used to sample soft tissue masses within the urinary bladder and is performed with retrograde placement of a red rubber catheter through the urethra to the level of the mass with ultrasound guidance. When at the level of the mass, a 20-mL syringe should be attached to the catheter using a Christmas tree adapter, and suction should be applied, drawing the mass into the catheter and occluding the flow of urine. With suction maintained, the catheter can be removed and a sample pulled into the syringe. Traumatic catheterization can be painful. Patients should thus be sedated or anesthetized as indicated.
Ultrasonography of the urinary bladder should be performed with an 8- to 10-MHz, microconvex, curvilinear probe or a 10- to 12-MHz, linear probe. American College of Veterinary Radiology and European College of Veterinary Diagnostic Imaging consensus statements for standardization of abdominal ultrasonography indicate imaging of the urinary system should include the urinary bladder, right and left kidneys, ureters (if visible), and urethra.5 According to the author, the region of the sublumbar lymph nodes should also be evaluated for metastatic disease. The urinary bladder should be imaged in both the transverse and longitudinal planes to evaluate the apex to the level of the urethra.4 Urinary bladder wall thickness can be measured but varies depending on patient size and volume of urine in the bladder.6 Urinary bladder wall thickness of <3 mm with a smooth margin is normal; the wall appears thicker as the bladder empties.
Hematuria is common in dogs and cats, but differentiation of the causes (eg, cystitis, calculi, neoplasia) can be difficult with radiography. Ultrasonography has better contrast resolution for differentiating fluid and soft tissue compared with radiography.
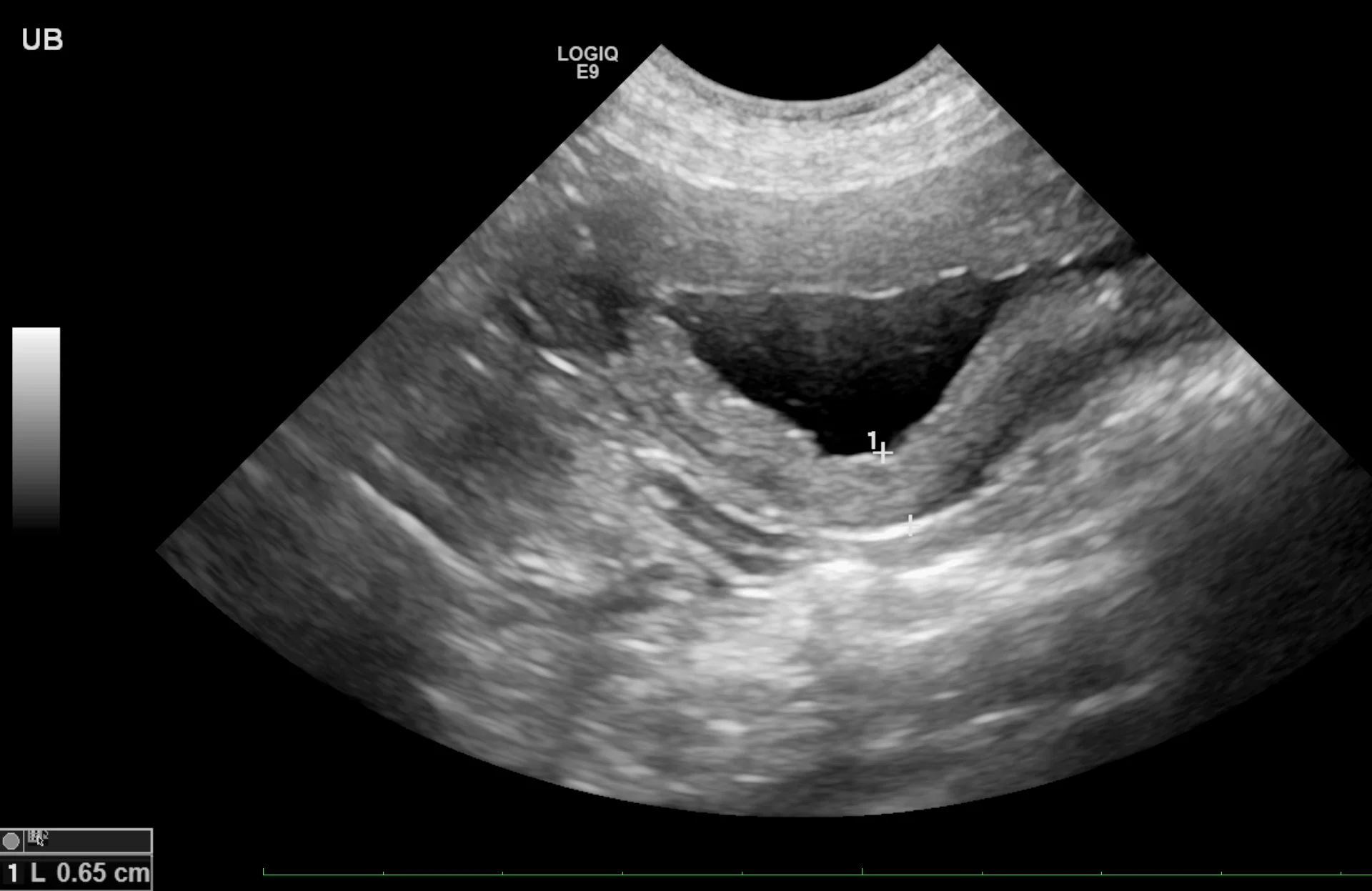
Cystitis
Cystitis is generally a diagnosis of exclusion, especially in cats with hematuria or difficulty urinating (ie, stranguria). Sterile and bacterial cystitis cannot be differentiated on ultrasound, and cystocentesis is needed to further characterize sediment even if hyperechoic foci are identified. The urinary bladder wall may be mildly thick or irregular on the luminal side (Figure 1). When measuring the wall of the urinary bladder, a representative region should be selected and the measurement made from the bladder mucosa to the serosa layer. Care should be taken to remain perpendicular to the wall, not to the ultrasound probe, to minimize measurement enlargement that can occur with obliquity. Overall measurement is more important for repeat evaluation to determine whether the urinary bladder is increasing or decreasing in size; retaining a consistent amount of urine (or catheterizing the patient and adding sterile saline) can help ensure consistent measurements.

Sagittal image of the urinary bladder with a diffusely thick wall due to cystitis. Left, cranial; bottom, dorsal
With chronic cystitis, a pedunculated mass can extend into the lumen of the urinary bladder, generally with a thin stalk that differentiates this polyp from a broad-based neoplastic mass. Urinary bladder neoplasia can also be primarily luminal and occupy a majority of the urinary bladder. Obtaining a sample may therefore be needed to differentiate neoplasia from cystitis. In addition, a neoplastic mass can result in a secondary infection, and 2 disease processes may be occurring. Repeat ultrasonography can help monitor patients with chronic cystitis.
Patients with chronic polypoid cystitis (Figure 2) can also have multiple projections of soft tissue into the lumen of the urinary bladder. Identification of these protrusions and histopathology, in addition to clinical signs of recurrent UTI or results of traumatic catheterization, are needed to differentiate polypoid cystitis and neoplasia.

Sagittal image of the urinary bladder with soft tissue extending into the lumen, indicating a presumed polyp in a 12-year-old spayed bichon frise crossbreed. Color flow Doppler shows the single vessel extending into the lesion. Left, cranial; bottom, dorsal
With diabetes mellitus, glucosuria can result in a secondary bacterial infection that can lead to an anerobic opportunistic infection that can cause gas within the wall of the urinary bladder (ie, emphysematous cystitis; Figure 3). If the gas is severe, reverberation artifact may inhibit complete evaluation of the urinary bladder.

Sagittal image of the urinary bladder with hyperechoic foci in the wall (arrow) with a reverberation artifact present distally, indicating gas within the wall in a 12-year-old neutered male bichon frise. Left, cranial; bottom, dorsal
Urinary Calculi
Urinary calculi are hyperechoic to the urine and can cast a distal acoustic shadow (ie, black streak under each calculus), regardless of the composition. In the author’s experience, increased power of the ultrasonography beam and advanced ultrasonography technology designed to minimize shadowing allow calculi to be at least 3 mm prior to creating a shadow. Lack of a distal acoustic shadow on small, gravity-dependent, hyperechoic foci does not therefore exclude diagnosis of sandlike calculi, although urinary sediment can have a similar appearance and can settle with gravity. Spatial resolution (ie, ability to distinguish 2 adjacent objects with the same echogenicity) is poor with ultrasonography, and multiple small calculi can appear as a single large calculus (Figure 4). Agitating the urinary bladder or repositioning the patient into sternal or lateral recumbency can help shift the calculi to determine the approximate length. In addition, measurements should be made parallel to the ultrasound beam for optimal accuracy; however, distal acoustic shadowing precludes these measurements. A reliable method of determining calculi type on ultrasound is not known.

Sagittal image of the urinary bladder with a collection of hyperechoic calculi (arrows) along the gravity-dependent dorsal border. Individual calculi cannot be seen due to poor spatial resolution. Echoic floating sediment, a nonspecific finding more common with higher-resolution probes, is present. Left, cranial; bottom, dorsal
Neoplasia
Urinary bladder masses (generally transitional cell carcinoma [Figure 5], but adenocarcinoma or rhabdomyosarcoma are also possible7-9) can cause hematuria or stranguria. On ultrasound, these masses are typically luminal and multilobulated, with rhabdomyosarcoma having a grapelike (ie, botryoid) appearance.7,9 Mineralized urinary bladder masses (rare) are usually urethral transitional cell carcinoma or prostatic adenocarcinoma that locally spreads into the urinary bladder. These masses can occupy the trigone region and occlude the ureters, causing secondary hydronephrosis (Figure 6).8

Sagittal image of the urinary bladder with 2 masses (arrows) along the ventral wall (one at trigone level) in a dog with transitional cell carcinoma. Left, cranial; bottom, dorsal

Sagittal image of the caudal aspect of the right kidney, showing severe dilation of the renal pelvis (arrows) and dilation of the ureter (circle) just caudal to the kidney caused by the transitional cell carcinoma seen in Figure 5. Left, cranial; bottom, dorsal
Ectopic Ureters
The cause of inappropriate urination can be difficult to diagnose, especially in young patients. Repeated radiography of the urinary system with an IV contrast medium (ie, excretory urethrography) or CT is the gold standard for diagnosis of ectopic ureters. In well-hydrated patients, furosemide (0.5-1 mg/kg IV) can be administered and the trigone region examined via ultrasonography for ureteral jets 2 to 3 minutes after administration.10 This is usually performed in the transverse plane to allow examination of the right and left ureteral papilla. Color flow Doppler can be used to examine the ureteral jets and assess whether only one jet is present or a jet is retrograde from the urethra. Furosemide generally causes the ureters to dilate to ≈2 mm. Ureters can then be tracked from the kidney to the urinary bladder or into the urethra if ectopic. It may be difficult to differentiate intramural and extramural ectopic ureters, although the latter are rare in dogs.10 Ability to follow the ureters depends on user expertise; not identifying ectopic ureters on ultrasound does not therefore exclude diagnosis.
Sampling
The most common sampling procedure using ultrasonography in the urinary system is ultrasound-guided cystocentesis, which is a sterile method to obtain urine (Figure 7). The same ultrasound probes and a 22-gauge, 1- to 1.5-inch (25- to 38-mm) needle should be used. Ideally, the needle should be directed toward the cranial or midbody region of the urinary bladder, carefully avoiding the trigone region. The site where the needle will enter the skin should be cleaned of any gel and wiped with alcohol. The needle should be placed either cranial or caudal to the ultrasound probe at a 45- to 60-degree angle to the patient and inserted approximately one-fourth of the needle length into the caudoventral abdomen, allowing the needle to be identified in the subcutaneous tissue prior to advancement into the urinary bladder. Once visible, the needle can be advanced into the urinary bladder and a sample obtained. If a mass is present, this technique can be used to collect a sample for cytology; however, there is a reported risk for seeding the abdomen.11 In this study, the needle tract was found during necropsy. Although risk for seeding the body wall is minimal, pet owners should be warned about the possibility. Traumatic catheterization can be performed instead to obtain a sample and avoid seeding the body wall.11

A needle (arrows) entering the urinary bladder from the cranial aspect and extending caudodorsally for cystocentesis. Left, cranial; top, ventral
Conclusion
Imaging the urinary bladder and urinary system to include the kidneys and sublumbar lymph nodes can be performed with minimal practice. Ultrasound results can be difficult to interpret due to numerous possible disease processes that may appear similar, but ultrasonography can help monitor progression, assess prognosis, and evaluate for evidence of obstruction that cannot be accomplished without more invasive procedures.