In the Literature
Gouvêa FN, Vargas AM, Guimarães EC, et al. Association between post-ACTH cortisol and trilostane dosage in dogs with pituitary-dependent hypercortisolism. Domest Anim Endocrinol. 2024;89:106871. doi:10.1016/j.domaniend.2024.106871
The Research …
Trilostane, a competitive inhibitor of 3-beta-hydroxysteroid dehydrogenase, has been the most common therapy for pituitary-dependent hyperadrenocorticism in dogs for >20 years. In addition to decreasing cortisol, trilostane decreases aldosterone, although secondary hyperkalemia is rare.1 Trilostane therapy increases endogenous ACTH and causes adrenal gland hyperplasia.2 Clinical studies support divided administration regimens as a safer alternative to the once-daily dose (2.2-6.7 mg/kg PO) that was initially approved.3-5
The authors of this retrospective study hypothesized that dogs with higher post-ACTH cortisol concentrations (cpACTH) during an ACTH stimulation test performed at the time of diagnosis would require higher trilostane maintenance doses. Cases (n = 43) that met the criteria of the ACVIM consensus statement on canine hyperadrenocorticism were identified,6 and dogs with cpACTH above the median (27 micrograms/dL) were compared with those below the median (Figure).
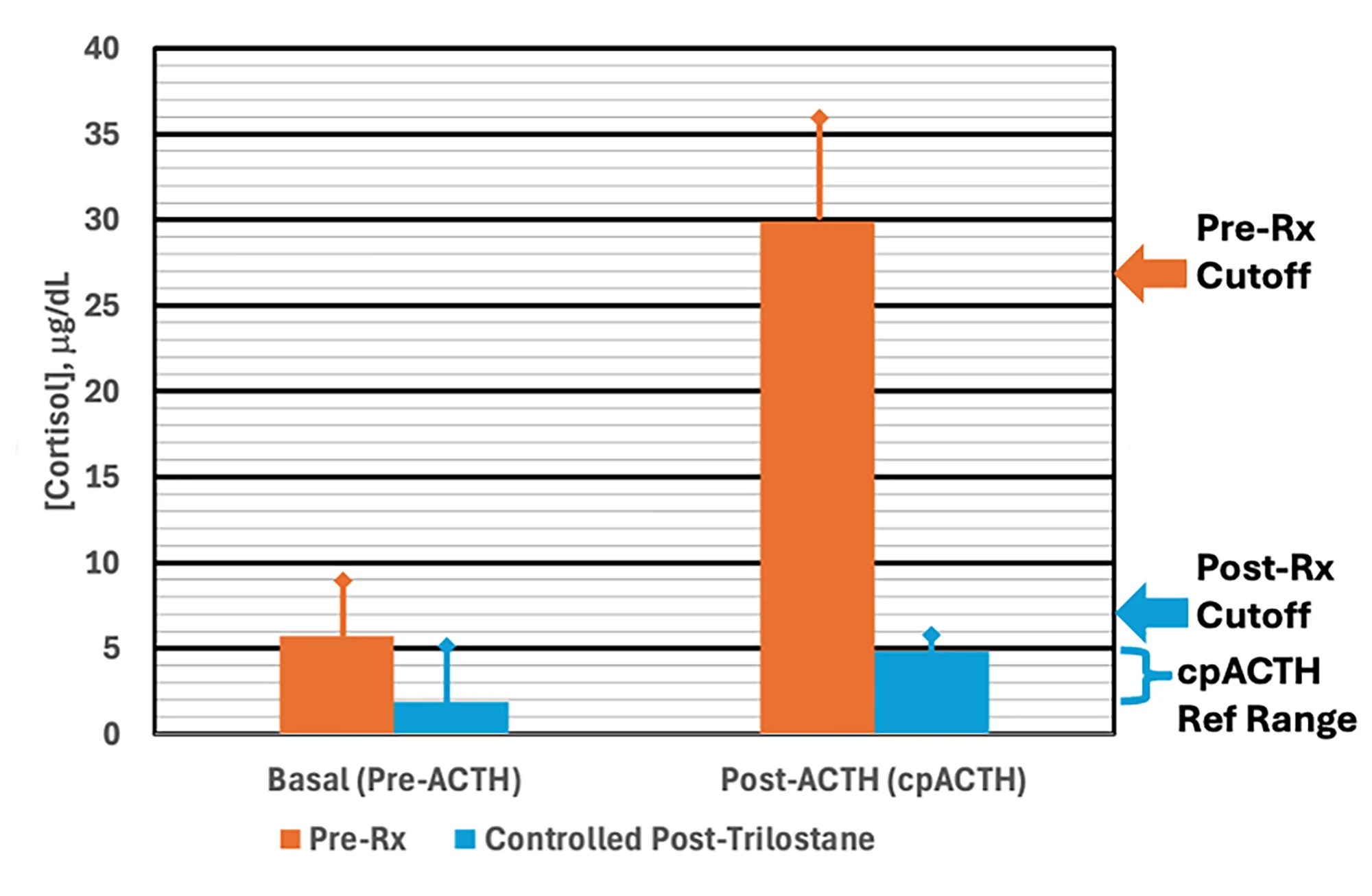
Basal cortisol concentrations and cpACTH before (orange) and after (blue) trilostane therapy
a Pre-Rx cutoff, median concentration of pretherapy cpACTH; post-Rx cutoff, highest observed posttherapy cpACTH
b Multiply by 27.6 to convert cortisol concentrations in micrograms/dL to the SI unit, nmol/L.
Initial doses of commercially available trilostane (n = 30) or compounded trilostane (n = 13) were 0.5 to 1 mg/kg PO every 12 hours. ACTH stimulation testing was performed every 4 to 6 weeks. Hyperadrenocorticism was considered controlled when cpACTH was ≤7 micrograms/dL, clinical signs resolved, and laboratory abnormalities normalized. An average of 3 recheck ACTH stimulation tests was required, and no adverse effects were observed.
Dose escalation was required in 88.4% of cases, and median final doses (commercially available trilostane, 2.5 mg/kg; compounded trilostane, 1.6 mg/kg) significantly exceeded median initial doses (commercially available trilostane, 0.6 mg/kg; compounded trilostane, 0.73 mg/kg). The median final dose was significantly higher for commercially available trilostane compared with compounded trilostane. Dogs above the median cpACTH were ≈2 times as likely to eventually require a higher dose than dogs below the median; however, the authors maintained that the same initial dose regimen is recommended, despite the increased likelihood of additional time needed to achieve optimal control.
Compounding was performed by a single pharmacy in this study. Prior studies with samples from multiple compounding pharmacies have found that ≈40% of compounded trilostane did not meet regulatory standards for potency and the actual content varied from 39% to 153% of the labeled content.7 This may explain the product differences noted in the current study.
… The Takeaways
Key pearls to put into practice:
Trilostane therapy that starts with 0.5 to 1 mg/kg PO every 12 hours and gradually increases in dose is recommended for treatment of pituitary-dependent hyperadrenocorticism in dogs to minimize signs of hypoadrenocorticism.
Periodic (ie, every 4-6 weeks) ACTH stimulation testing is recommended to monitor trilostane therapy. cpACTH >7 micrograms/dL, particularly when noted concurrently with unresolved clinical signs, indicates a dose increase is warranted. Dose escalation is common, possibly due in part to adrenal hyperplasia during therapy.
Pet owners should be informed that cpACTH ≥27 micrograms/dL at the time of diagnosis indicates the trilostane dose will likely need to be gradually increased to achieve disease control. As basal (ie, pre-ACTH) cortisol had no predictive value in this study, basal cortisol measurement may be eliminated as a cost-savings measure, especially in patients without signs of hypoadrenocorticism.
Most studies have evaluated dosage and efficacy of commercially available trilostane. Greater variability should be expected from compounded trilostane.
You are reading 2-Minute Takeaways, a research summary resource presented by Clinician’s Brief. Clinician’s Brief does not conduct primary research.