Tracheal Collapse in Dogs
The trachea is composed of 35 to 45 cartilaginous, C-shaped rings joined dorsally by the trachealis muscle, mucosa, and connective tissue (dorsal tracheal membrane; see Trachea Facts).1,2
Related Article: Tracheal Collapse: Extraluminal Rings & Tracheal Stents
Tracheal collapse is progressive, dorsoventral flattening of the tracheal lumen. It is most common in middle-aged, small-breed dogs (eg, Yorkshire terrier, toy poodle, Pomeranian, Chihuahua, pug)2 but has also been reported in large-breed dogs, ponies, cows, cats, and a goat.3-7 Initially, laxity of the trachealis muscle results in coughing and noisy breathing as the dorsal tracheal membrane billows in and out of the tracheal lumen with each breath. As the condition progresses, the cartilaginous rings become more ovoid and the distance between them increases, resulting in dorsoventral flattening of the trachea and increasingly severe episodes of coughing and exercise intolerance. The tracheal lumen diameter may become so narrow that the lumen is nearly obliterated, leading to respiratory distress and collapse.
The cause of tracheal collapse is unknown but is thought to be a combination of environmental and genetic factors.2 Histologically, cartilaginous rings from affected dogs are hypocellular with decreased glycoprotein and glycosaminoglycan and subsequent reduced water retention.8-10 Obesity, pollutants, environmental allergens, and kennel cough may be associated with disease progression.2,10,11
Signs of tracheal collapse include coughing (eg, a goose honk); noisy breathing; and, in severe cases, dyspnea, cyanosis, and hyperthermia. Coughing episodes may increase with excitement, tracheal pressure (eg, from a leash or collar), exercise, eating, or drinking. A cough may be elicited by palpating the trachea at the thoracic inlet, although tracheal collapse was successfully diagnosed in this manner in only 41% of affected dogs in a previous study.12 In some dogs, it is possible to palpate flattened cartilages along the cervical trachea. Hepatomegaly is another common concurrent finding, but the association is unclear.1
Related Article: Intermittent Exercise Intolerance
Diagnosis
Figure 1. Lateral thoracic radiograph of a dog with tracheal collapse. The most severe area of collapse is just caudal to the thoracic inlet.
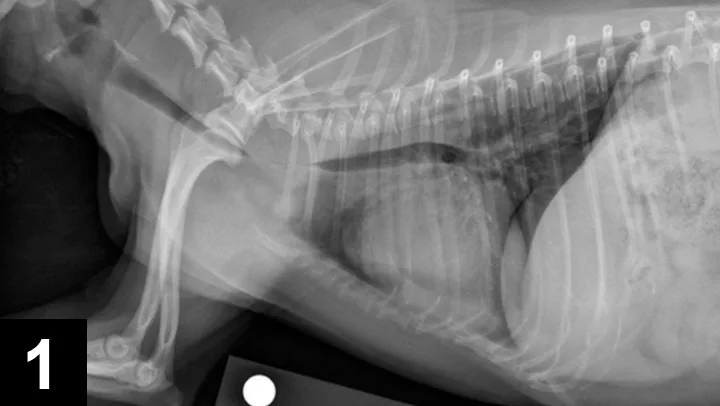
Definitive diagnosis is based on imaging (eg, survey radiography, fluoroscopy, ultrasonography, computed tomography, tracheobronchoscopy).13 Survey radiography should include dorsoventral and lateral views of the cervical region and thorax (Figure 1). Collapse of the trachea is best viewed in the cervical region during inspiration and in the intrathoracic region during exhalation.1 Radiography is critical to rule out conditions that may cause similar signs (eg, intrathoracic masses, pleural effusion) and cardiovascular abnormalities (eg, heart enlargement) that may complicate treatment. Radiography is noninvasive, cost effective, widely available, and can be performed without the risks associated with general anesthesia; however, false-positive results have been reported in 25% of dogs,14 and sensitivity ranges from 60% to 90%.2 In comparison, fluoroscopy allows direct viewing of tracheal motion during all phases of respiration, is noninvasive, and is very sensitive, although false-positive findings have also been reported with fluoroscopy.14 In one study, radiography underestimated the severity and frequency of collapse as compared with fluoroscopy.15
Tracheoscopy, the gold standard for diagnosing tracheal collapse, enables direct viewing of the trachea and mainstem bronchi, quantification of severity and extent of collapse, identification of concurrent inflammation, and collection of tracheal or bronchial samples for culture and cytology. With tracheoscopy, tracheal collapse can be categorized based on the Tangner and Hobson grading system with grades I, II, III, and IV characterized by 25%, 50%, 75%, and 100% collapse, respectively16 (Figure 2). Disadvantages include limited availability, cost, and need for general anesthesia. Because veterinary patients may be small, tracheoscopy is often performed under injectable anesthesia and without intubation; as such, ventilation cannot be assisted during the procedure, and oxygen must be supplemented through the endoscope or with an intratracheal catheter. Some dogs with severe tracheal collapse develop dyspnea and cyanosis during anesthetic recovery.
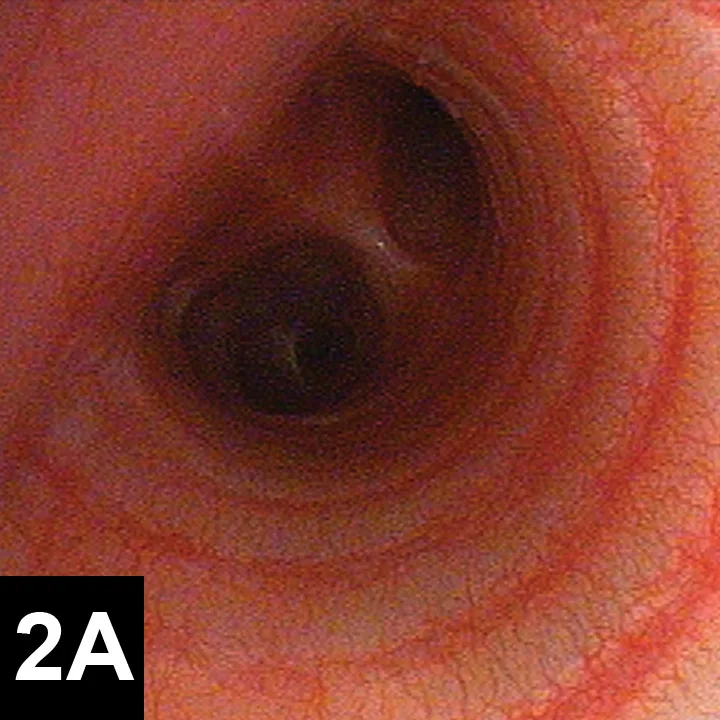
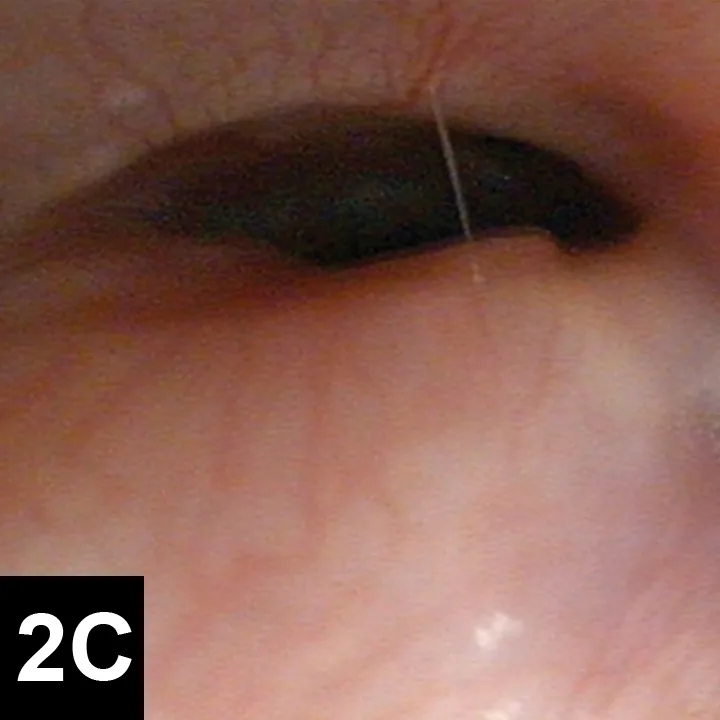
Figures 2. Endoscopic view of tracheal collapse: Grades I–IV (A-D) of tracheal collapse, respectively. Used with permission.23
Medical Management
Most dogs with tracheal collapse are managed medically; in one study of 100 dogs, the success rate with medical management alone was 71%.10 Dogs presenting with acute respiratory distress are administered flow-by oxygen and mild sedation. Long-term treatment for mildly to moderately affected dogs includes oral antitussives and tapering doses of corticosteroids.17,18
Concurrent respiratory infections are treated with antibiotics based on culture and sensitivity testing of tracheal wash or brush samples. More than 80% of dogs with tracheal collapse test positive on bacterial culture16 (more commonly Pseudomonas spp; less commonly, Enterobacter spp and Citrobacter spp).19 Bacteria cultured from tracheal samples may not be pathogenic, but infection should be suspected if supported by neutrophilic inflammation on cytology.19 If antibiotics are chosen empirically, doxycycline, cephalexin, or amoxicillin–clavulanate are generally effective.17
Bronchodilators or antihistamines may also be prescribed. No clinical trials currently demonstrate the safety and efficacy of bronchodilators in medical management of tracheal collapse, but their use can be justified in patients with concurrent lower airway disease.17 In one study, 13 of 14 dogs with tracheal collapse were treated with the anabolic steroid stanozolol and demonstrated improvement.20
In obese patients, weight loss is critical and can produce dramatic improvement. Other adjuncts include limiting tracheal pressure (using a harness instead of a collar) and limiting exposure to respiratory irritants (eg, smoke, dust, other particulate matter).
Structural Support
Prosthetic support of the trachea—placement of extraluminal tracheal rings or an intraluminal stent—is recommended when medical management fails. Extraluminal rings expand the lumen diameter and prevent collapse from negative airway pressure within the trachea and mechanical forces external to the trachea. Rings are placed around the cervical trachea through a ventral midline approach and the intrathoracic trachea through a lateral thoracotomy at the third intercostal space. Dissection of neurovascular structures along the trachea is required for ring placement, and associated complications can be life threatening.2,12,16
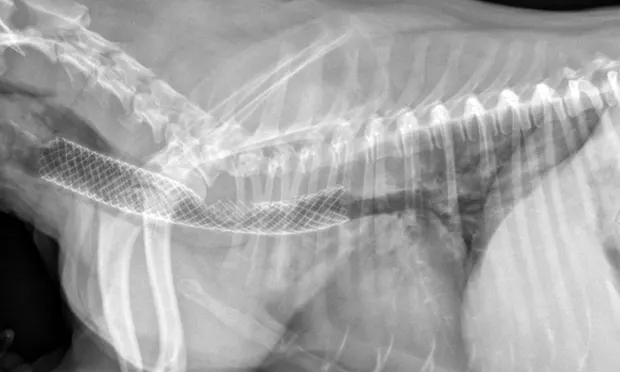
Figure 3A. Fully deployed stent. Courtesy of Infiniti Medical.
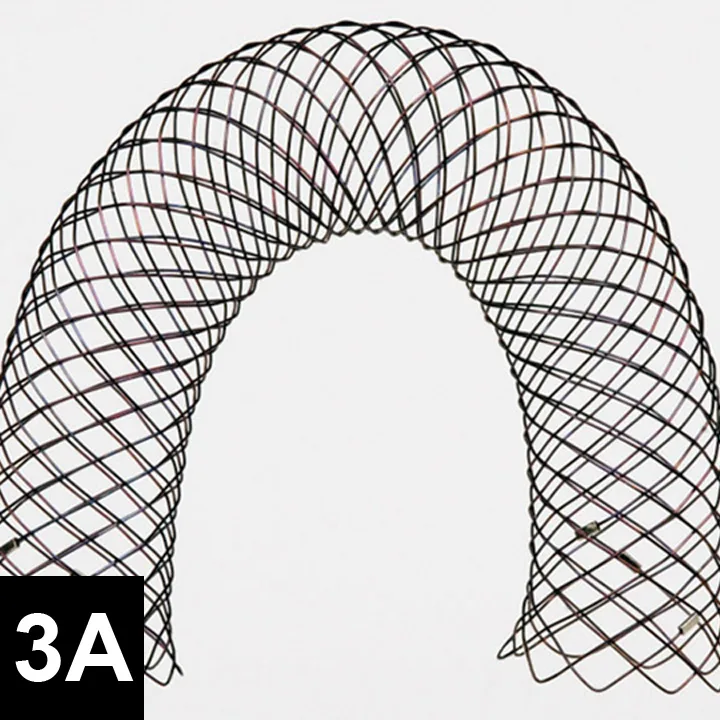
Damage to the recurrent laryngeal nerves resulted in laryngeal paralysis in 11% to 30% of dogs that underwent extraluminal, peritracheal placement of ring or spiral prosthetic devices in previous studies.12,16 Damage to segmental blood supply can result in partial- or full-thickness tracheal necrosis.21,22 Signs include coughing and subcutaneous emphysema; death is possible. Pneumothorax is another severe complication reported with placement of extraluminal cervical prosthetics.2 Surgeons should be prepared to place a thoracostomy tube intraoperatively.
Figure 3B. Partially deployed stent. Courtesy of Infiniti Medical.
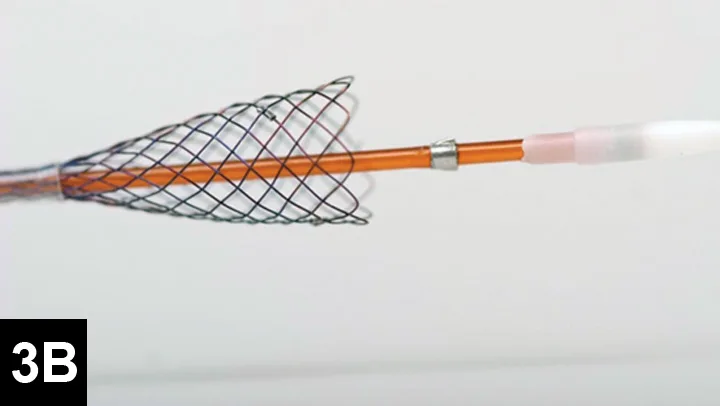
Many surgeons prefer intraluminal stents because they can be noninvasively placed in the cervical or thoracic trachea, reducing risk for complications and shortening anesthetic times. Vet Stent–Trachea is a woven, reconstrainable, self-expanding, nitinol stent3-8 (Figure 3). Nitinol, a nickel–titanium alloy, has thermal shape memory, super elasticity, and elastic hysteresis; the latter minimizes outward force on the interior lumen of the trachea, regardless of stent size.24 The undeployed intraluminal stent is secured within a low-profile delivery system that has radiopaque markers to facilitate positioning. As it is released from the catheter, the stent expands to meet the internal wall of the trachea, foreshortening as it increases in diameter. Because the stent is reconstrainable, it can be pulled back into the delivery system for repositioning after partial release.
The flexibility of woven nitinol stents allows them to maintain their cylindric shape along the length of the trachea, despite changes in tracheal direction or diameter. Radial stress applied to the interior lumen of the trachea prevents migration of the stent, as long as an appropriate size is chosen.2,26
Preferred stent size is estimated from survey radiographs taken with the patient under general anesthesia. A probe marked with radiopaque lines at 1-cm increments is placed within the esophagus to permit correction associated with potential magnification. The endotracheal tube is retracted to where the cuff is inflated within both the cricoid and thyroid cartilages, and the trachea is expanded with positive pressure to determine maximal lumen diameter. Length is measured from the caudal surface of the cricoid cartilage to the cranial edge of the carina. Tracheobronchoscopy can be used to recheck the length of the trachea, determine the grade of collapse, and obtain samples for culture and cytology.
Stent selection is based on matching the desired stent diameter and length with measurements in the manufacturer’s foreshortening chart, which provides an estimate of final length based on the predicted diameter of expansion. The stent should span from just caudal to the cricoid cartilage to just cranial to the tracheal bifurcation. Final stent width should exceed the maximal diameter of the trachea by 10% to 20% to prevent stent migration.
Because dogs anesthetized for tracheal measurements usually have severe collapse, immediate stent placement is preferable. However, the cost of the stents limits the sizes most practices have on hand. If an appropriate size is not available, the clinician may attempt to recover the dog from anesthesia and manage it medically until the desired stent can be obtained. Dogs that do not recover well from anesthesia may require overnight ventilation or immediate placement of a less-than-ideal stent.
Step-by-Step: Stent Placement
Stent placement is performed with the patient under general anesthesia and is guided by fluoroscopy or tracheobronchoscopy. Tracheobronchoscopy with a rigid scope permits direct viewing of the airway and more precise stent placement.
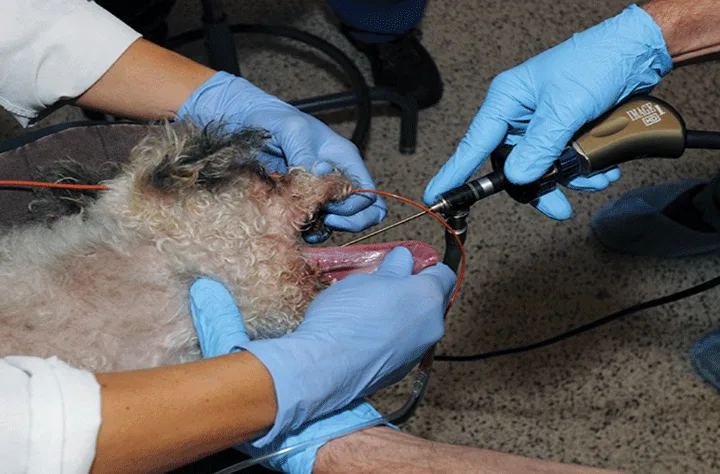
Step 1.
Place the dog in left lateral recumbency with its head and neck extended. Insert a 5-French red rubber catheter attached to an oxygen source transorally into the trachea; carefully insert a rigid scope alongside and advance it until the carina is visible.
Figure 1. Stent placement guided by tracheobronchosopy; the tracheal lumen is viewed with a rigid scope. Anesthesia is maintained with constant rate infusion, and oxygen is delivered into the trachea through a red rubber catheter.
Postsurgical Care & Follow-up
Patients are recovered in an oxygen cage and monitored for signs of respiratory distress. They are sent home on a tapering dose of corticosteroids, 2 weeks of antibiotics, and client instructions to administer sedatives and antitussives as needed. In one study of 18 dogs, the mortality rate was 11.1% within 60 days after stent placement; however, long-term improvement was observed in the remaining dogs.22 Stress, excitement, and exercise need to be limited for 4 weeks. Patients should be reevaluated (ie, examination, survey radiography) at 1, 3, and 6 months procedure. Because the stent initially irritates the airway, coughing is expected but must be controlled to prevent stent fracture (4**A, 4B) or granulation tissue formation (4**C) [see above]. Other potentially life-threatening complications include stent migration, tracheal rupture, and collapse of mainstem bronchi or nonstented regions of the trachea. Rare complications include rectal prolapse and perineal hernia.23 Most clients note immediate improvement in quality of life and, despite the progressive nature of the condition, are satisfied with the procedure.
This article is part of the WSAVA Global Edition of Clinician's Brief.
Mary Dell Deweese, DVM, is a veterinary student at University of Tennessee College of Veterinary Medicine with an interest in small animal medicine and surgery. This paper was authored as part of a summer research program for veterinary students, funded by the Center of Excellence in Livestock Diseases and Human Health at the University of Tennessee.
Karen M. Tobias, DVM, MS, DACVS, is professor of small animal soft tissue surgery at University of Tennessee and mentors veterinarians in writing educational articles. She taught at University of Georgia and Washington State University, authored Manual of Small Animal Soft Tissue Surgery, and was coeditor of Veterinary Surgery: Small Animal. She completed her internship and residency at Purdue University and The Ohio State University, respectively, and earned her DVM from University of Illinois. Dr. Tobias presented on essential wound care techniques at the NAVC Conference 2013.