Top 5 Peripheral Cranial Nerve Lesions in Dogs & Cats
Jonathan M. Levine, DVM, DACVIM (Neurology), Texas A&M University

Lesions affecting cranial nerve projections distant from the central nervous system (called peripheral cranial nerve lesions) are relatively uncommon. Neurologic examination represents a readily available, albeit imperfect, means to determine if a cranial nerve lesion is likely to be peripheral or central. Dogs and cats with peripheral lesions have normal postural reactions, mentation, and spinal reflexes. Unless the vestibulocochlear nerve is affected, discernible ataxia or paresis will not be present. In many cases, advanced imaging and additional diagnostic modalities are required to determine the location of a cranial nerve lesion and the likely underlying cause.
Following are 5 common disease processes that result in peripheral cranial neuropathies in dogs and cats.
1. Nerve Sheath Tumors
Nerve sheath tumors (NSTs) are perhaps the most common cause of chronic, progressive peripheral cranial nerve disease in older dogs. They arise from Schwann cells or pericytes, are typically slow growing, and are invasive to surrounding nervous system tissue. Metastasis is a rare event associated with NSTs. Whereas they most commonly affect the brachial plexus and lumbosacral plexus, NSTs also can arise within cranial nerves, with the trigeminal nerve most frequently affected. Clinical signs of trigeminal NSTs are ipsilateral to the NST and include unilateral masticatory muscle atrophy, facial hypalgesia, reduced corneal sensation, and Horner syndrome.1 Magnetic resonance imaging (MRI) is the modality of choice for identifying lesions. On MRI, NSTs are mass-like, tubular structures that are contiguous with a nerve (Figure 1).2 Lesions are often hyperintense (bright) on T2-weighted images (T2WIs), indicating high water content from edema, neoplastic cells, or inflammation, and strongly contrast enhance on T1-weighted images (T1WIs) following the delivery of gadolinium, suggesting a blood–nerve barrier breakdown or neoangiogenesis.
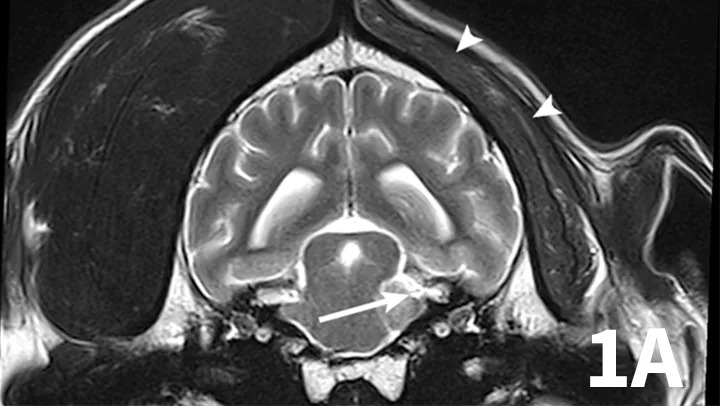
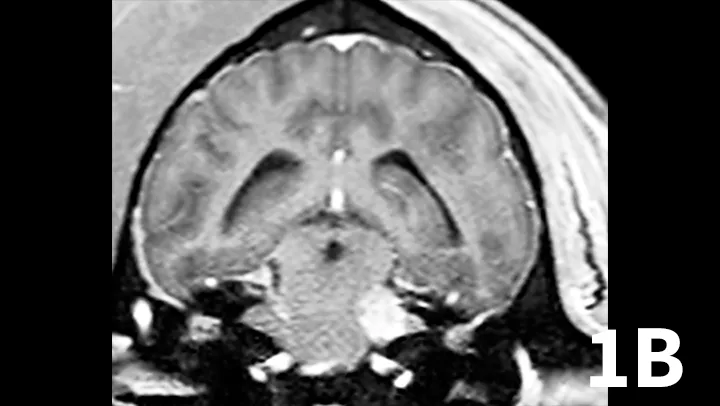
Transverse T2WI (A) and postcontrast T1WI (B) from a 10-year-old golden retriever with a 6-month history of left masticatory muscle atrophy. Adjacent to the left lateral pons is a mass-like lesion (arrow) confluent with the left trigeminal nerve that is T2-hyperintense and contrast-enhances on T1WI. There is profound atrophy of the left temporalis muscle (arrowheads), which is likewise T2-hyperintense with subtle contrast enhancement on T1WI. These findings are consistent with a nerve sheath tumor of the proximal trigeminal nerve with secondary neurogenic muscle atrophy.
Surgical removal may be feasible (depending on lesion location) but is not commonly pursued because of surgical risks and adjacent vital structures. Limited data exist regarding postoperative survival times.
Radiotherapy is commonly used to control tumor progression, but outcome data from large cohorts have not been published. Reported survival times in a cohort of 10 dogs with trigeminal NST receiving a variety of therapies ranged from 0 to 27 months (median, 8 months) post MRI.1
2. Otitis Interna
Inner ear infection (otitis interna) is the most common cause of peripheral vestibular disease in dogs.3 It typically results from extension of bacterial infection within the external ear canal through a ruptured tympanic membrane, or from the nasopharynx via the Eustachian tube. The most commonly identified pathogens in dogs include Staphylococcus spp, Streptococcus spp, Proteus spp, and Pseudomonas spp.4 In cats, Staphylococcus spp, Streptococcus spp, Pasteurella spp, and anaerobic bacteria are believed, in this author’s experience, to be the most commonly involved.
Animals may have erythema and pruritus affecting the external ear or pinna, odor or discharge from the ear, and pain in addition to signs of peripheral vestibular disease. Although diagnosis can be suggested by otoscopy, advanced imaging is superior, as it permits visualization of the osseous bulla and inner ear. The author typically prefers MRI to image dogs suspected of otitis interna because it is superior to computed tomography (CT) for detecting intracranial extension of lesions and detecting other causes masquerading as otitis interna. On MRI, bullae may appear thickened and are usually filled with T2-hyperintense/T1–iso-hypointense (dark) material; abnormal tissue within the bulla usually contrast-enhances T1WI (Figure 2). On T2WI, there may be a loss of signal within the inner ear. Myringotomy and deep ear flush are recommended to identify the underlying infectious agent and assist in removing debris or pus that will prohibit clearance of infection. Antimicrobial therapy is typically continued for 6 to 8 weeks, as bone infection is likely.
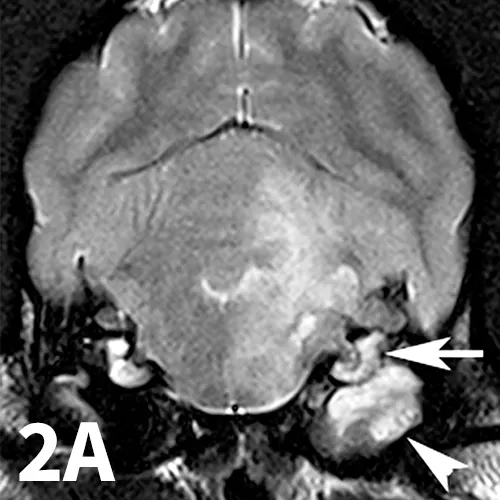
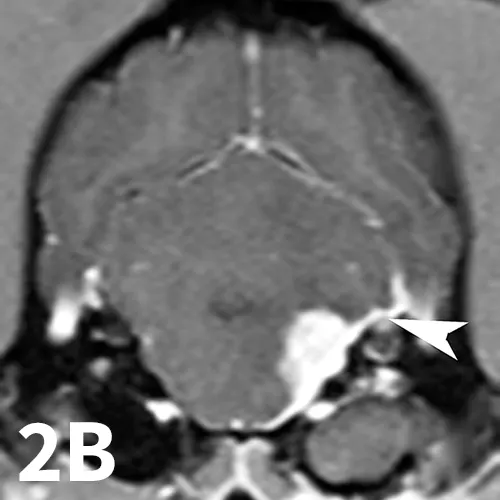
Transverse T2WI (A) and T1WI (B) from a 6-year-old cocker spaniel with a 6-month history of left peripheral vestibular disease that acutely worsened, with signs consistent with central localization. The left middle ear cavity is filled with T2-hyperintense material (arrowhead). The left inner ear is hypointense (arrow) compared with the right side. In addition, the left medulla and cerebellum contains patchy T2-hyperintense lesions. T1 postcontrast images show enhancement of the meninges (arrowhead) and a mass-like, enhancing lesion adjacent to the internal acoustic meatus. These findings are consistent with chronic otitis interna and media with secondary extension into the meninges and central nervous system.
Animals with recurrent or unsuccessfully treated otitis media or otitis interna may require bulla osteotomy. A total ear canal ablation may be required in animals with severe otitis externa or a stenotic ear canal.
3. Trigeminal Neuritis and Idiopathic Trigeminal Neuropathy
A range of infectious and noninfectious inflammatory diseases can affect peripheral nerve branches. The most frequently recognized inflammatory entity in dogs affecting peripheral branches of cranial nerves is trigeminal neuritis or idiopathic trigeminal neuropathy (ITN).5 Affected dogs typically have an acute onset of clinical signs secondary to bilateral motor branch dysfunction of the trigeminal nerves. This bilateral involvement of the trigeminal nerve results in inability to close the mouth (drop jaw), difficulty prehending food, and drooling.5 About 33% of dogs have sensory involvement of the trigeminal nerve, and <10% have Horner syndrome or facial nerve dysfunction.5 While the underlying pathogenesis of this disease is not known, it is believed to be noninfectious and inflammatory in origin.5 Other causes can be excluded via MRI and cerebrospinal fluid analysis. Reported MRI findings include bilateral, subtle enlargement of the trigeminal nerve with associated T2 hyperintensity (Figure 3).2 The reported mean time to recovery is 22 days with <10% of dogs taking >6 weeks to recover.5 No treatment beyond assisted feeding is recommended for ITN. It may be necessary to try several food consistencies to determine what will be easiest for a particular dog to prehend; rarely, feeding tubes may be necessary. Team members who handle dogs (or cats) with any acute neurologic disease process, especially involving the trigeminal nerves or lower motor neuron system, should wear gloves, as rabies is a differential diagnosis, especially in unvaccinated animals.
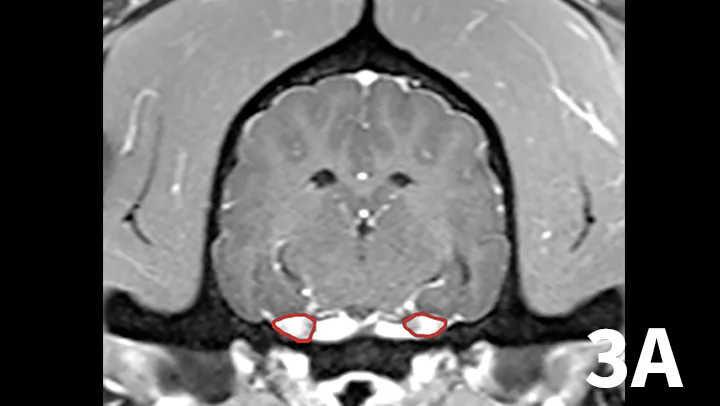
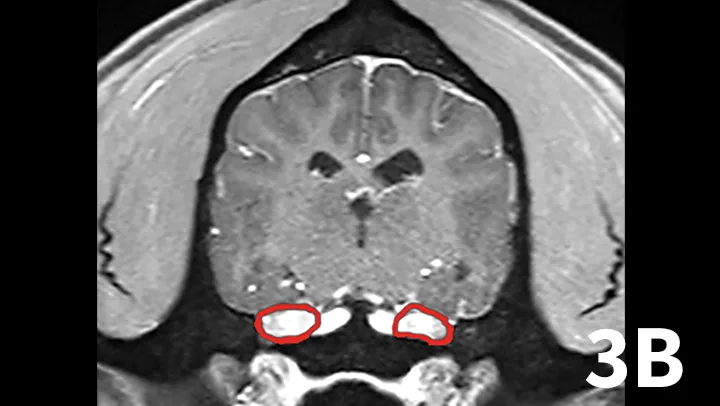
Transverse postcontrast T1WI at the level of the caudal thalamus from a normal dog (A) and a dog with trigeminal neuritis (B). The trigeminal nerves are outlined in each study in red. The dog with trigeminal neuritis has grossly enlarged trigeminal nerves without an overt, identifiable mass.
4. Idiopathic Vestibular Syndrome
Idiopathic vestibular syndrome occurs in older dogs and cats of any age. It results in acute onset, unilateral vestibular dysfunction, without concurrent Horner syndrome or facial nerve signs. The underlying etiopathogenesis is unknown, but possibilities include transient viral infection similar to vestibular neuritis in humans or disturbance in endolymph flow, as seen in Ménière’s disease in humans.6 Advanced diagnostics, including MRI, cerebrospinal fluid analysis, and infectious disease testing for Toxoplasmagondii (dog, cat), Neosporacaninum (dog), fungal causes, rickettsial organisms, and canine distemper virus (dog) are unremarkable. Most animals recover rapidly (within a few days to a week), although up to 3 to 4 weeks may be required in severe cases. Recurrent episodes of idiopathic vestibular syndrome are rarely reported.7 No specific treatment is recommended. Medications that mitigate vertigo, such as maropitant and meclizine, can be used to treat nausea and dizziness associated with this disease.
5. Idiopathic Facial Nerve Paralysis
Approximately 50% of dogs with peripheral facial nerve weakness are diagnosed with idiopathic facial nerve paralysis.7 Signs are often relatively acute in onset. Cocker spaniels are believed to be overrepresented, and affected dogs are usually older than 5 years.7 Dysfunction may be bilateral or unilateral. The underlying etiopathogenesis of idiopathic facial nerve paralysis in dogs is unknown but has been speculated to involve immune-mediated neuritis or viral infection. Diagnostics, including MRI, cerebrospinal fluid analysis, and testing for hypothyroidism and infectious disease, are unremarkable. Treatment (eg, eye lubrication) is supportive, with a guarded prognosis for return to function.
CT = computed tomography, ITN = idiopathic trigeminal neuropathy, NST = nerve sheath tumor, MRI = magnetic resonance imaging