Top 5 Causes of Passive Cervical Flexion
Mark Troxel, DVM, DACVIM (Neurology), Massachusetts Veterinary Referral Hospital, Woburn, Massachusetts

Cervical flexion is a common abnormal body posture that can be either active or passive. Patients with active cervical flexion hold their head down and are reluctant to lift it, often as the result of neck pain. Patients with passive cervical flexion are unable to fully lift or hold up their head because of muscular weakness. Passive cervical flexion is more common in cats, as cats lack a nuchal ligament (ie, a ligament that helps sustain the weight of a dog’s head and keep it elevated).
Following are 5 of the most common causes of passive cervical flexion in cats and dogs (for additional differential diagnoses in cats, see Additional Differential Diagnoses for Passive Cervical Flexion in Cats).
1. Hypokalemic Myopathy
Hypokalemic myopathy has been reported secondary to several disorders1-11 (see Common Causes of Hypokalemia) and can occur in cats and dogs of any age but is more common in older animals.3,5 Patients are often presented with stiff–stilted gait, weakness, exercise intolerance, cervical flexion (especially in cats), and muscle pain.1-11 Cervical flexion due to hypokalemia (see Video) is common in cats but uncommon in dogs.1 Delayed to absent postural reaction deficits and reduced patellar and withdrawal reflexes may be present depending on the severity of weakness. Muscle pain is occasionally noted.
Diagnosis in patients with compatible clinical signs of hypokalemia (<3.5 mEq/L) is relatively straightforward.2-6 Serum creatine kinase (CK) levels are often moderately to markedly elevated.2-6 Patients with chronic kidney disease (CKD) are likely to have elevated BUN and creatinine levels with concurrent isosthenuria, and although CKD can be a separate differential diagnosis for cervical flexion, it is often a result of potassium loss in the urine. Thoracic radiography and abdominal ultrasonography can be used to identify signs of CKD, adrenal neoplasia (eg, aldosterone-secreting tumor), and metastatic disease. Electrodiagnostic testing and nerve and muscle biopsy can be performed but are often unnecessary, except in complicated cases.
Treatment consists of potassium supplementation and correction (eg, adrenalectomy) of the underlying cause. Patients with moderate to severe hypokalemia (<3 mEq/L) should be administered potassium chloride in IV fluids—not to exceed 0.5 mEq/kg/hour except in life-threatening circumstances—and ECG should be closely monitored. Dogs and cats with mild hypokalemia or potassium levels that have been normalized with IV fluid supplementation should be administered potassium gluconate (2 mEq/4.5 kg body weight PO with food every 12 hours, adjusted as necessary).6 The dose should be titrated as indicated by serial potassium levels. Hypokalemia-induced cervical flexion tends to resolve quickly with potassium supplementation. Overall long-term prognosis is variable and depends on the inciting cause of hypokalemia.
COMMON CAUSES OF HYPOKALEMIA1-12
CKD with excessive urinary potassium loss
Diabetic ketoacidosis (possibly through osmotic diuresis, correction of acidosis, or insulin-mediated cell uptake)
Insufficient dietary intake (eg, diet with insufficient potassium; Figure 1)
GI loss
Diuresis after resolution of urethral obstruction
Hypokalemia secondary to loop or thiazide diuretics
Mineralocorticoid excess (eg, aldosterone-secreting adrenal tumor)
Congenital hypokalemia (in Burmese cats)
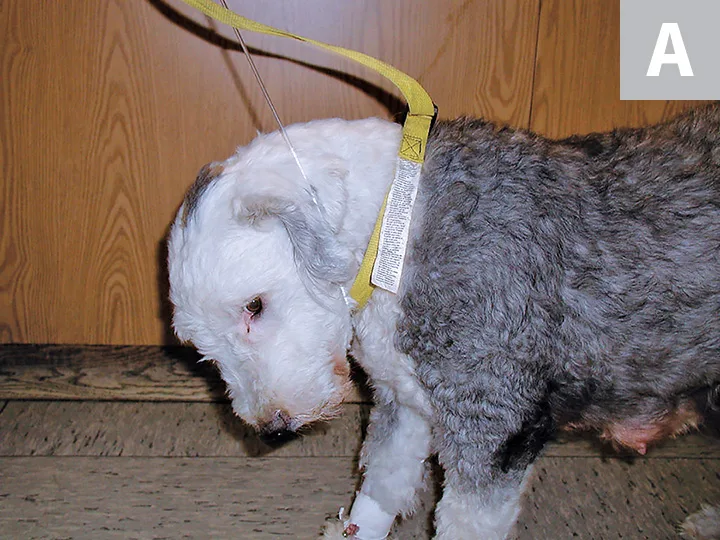

Passive cervical flexion in a 3-year-old postparturient female Old English sheepdog with hypokalemia secondary to poor nutrition (A). Clinical signs resolved with potassium chloride supplementation in IV fluids (B).
2. Thiamine Deficiency
Thiamine (ie, vitamin B1) is a water-soluble, heat-labile vitamin that is crucial to carbohydrate metabolism. Dogs and cats are unable to endogenously synthesize thiamine, so it must be obtained from the diet.12-14 Although the risks for thiamine deficiency are well-known, inadequate supplementation can result from manufacturing or storage errors; during manufacturing, thiamine can be easily destroyed by high temperatures, chlorinated water, and/or being in a neutral or alkaline state. Manufacturers must compensate for this potential loss by adding high levels of thiamine to food. In addition, thiamine content declines with long-term storage because of the combined effects of heat and humidity. A recent report described 17 cats with thiamine deficiency that were fed the same commercial dry cat food.15
Thiamine deficiency can occur in dogs and cats of all ages and breeds. Thiamine deficiency is often seen in dogs with severe or end-stage liver dysfunction because the liver is a storage center for thiamine. Cats are more commonly affected than dogs due to their 3-fold higher dietary thiamine requirement (see Common Dietary Causes of Thiamine Deficiency).16 Clinical signs in cats typically include nonspecific signs of inappetence, weight loss, vomiting, and diarrhea, as well as neurologic signs of generalized weakness, cervical flexion (Figure 2), central vestibular dysfunction, and dilated pupils.17,18 Clinical signs in dogs include nonspecific signs of depression, inappetence, weight loss, vomiting, mental dullness, weakness, and seizures.13 Severe untreated thiamine deficiency can lead to coma and death in both species.

Passive cervical flexion in a cat with thiamine deficiency
Definitive diagnosis is difficult because several forms of thiamine exist in the body and no single laboratory test measures all forms. A presumptive diagnosis is typically made based on compatible clinical signs, history of dietary changes (as described), and response to treatment. Routine laboratory test (eg, CBC, serum chemistry profile, urinalysis) results are usually normal.12 MRI performed in patients with CNS signs may demonstrate bilaterally symmetrical T2-weighted hyperintense lesions in the lateral geniculate nucleus of the thalamus, caudal colliculi in the midbrain, and/or vestibular nuclei in the medulla.19,20 CSF is usually normal.
Therapy should be focused on thiamine replacement and feeding a high-quality diet rich in thiamine. Limited data exist regarding an ideal dose, but ranges of 25 to 150 mg for cats and 100 to 600 mg for dogs have been described17-23; daily IM thiamine injections for 3 to 5 days are recommended, followed by PO supplementation for 2 to 4 weeks.17-23 Resolution of cervical flexion may take up to several weeks, but prognosis is good to excellent if the condition is diagnosed and treated early.
COMMON DIETARY CAUSES OF THIAMINE DEFICIENCY12-15,21,22,46,47
Noncommercial diet with inadequate thiamine supplementation
Flesh and viscera from raw fish or shellfish containing thiaminases that break down thiamine
Overly heated food
Sulfur dioxide- or sulfite-preserved foods
3. Hyperthyroidism
Hyperthyroidism is the most common endocrinopathy of middle-aged and older cats and is most often the result of excessive circulating levels of thyroxine (T4) and triiodothyronine (T3) produced by a thyroid adenoma or hyperplastic adenomatous thyroid tissue.24-28 Thyroid carcinoma as a cause of hyperthyroidism is rare.24
Clinical signs include weight loss (despite polyphagia), hyperactivity, polyuria/polydipsia, unkempt hair coat, GI signs (eg, vomiting, diarrhea), panting/dyspnea, lethargy or mental dullness, neuromuscular weakness, anxiety, nervousness, and/or behavior changes. Physical examination may reveal a thin cachectic cat, a palpable thyroid nodule, a heart murmur, tachycardia, arrhythmia, tachypnea, alopecia or unkempt hair coat, aggression, or mental dullness.24-28 Cervical flexion and generalized muscle weakness occur in some cats with hyperthyroidism. Hyperthyroidism can also cause a vascular lesion in the cervical spinal cord of some cats that results in cervical flexion. In a study, concurrent hypokalemia was reported in 4 hyperthyroid cats with cervical flexion,29 but the cause of hypokalemia was undetermined.
Diagnosis for most cats is made through measurement of elevated total T4 (TT4) by radioimmunoassay, but ≤10% of hyperthyroid cats have normal TT4 during initial testing; these cats are in the early stage of disease and either TT4 has not risen above the high-end normal limit or nonthyroidal illness is artificially lowering the TT4 level.24,25,30-33 Free T4 should not be used as the sole screening test for hyperthyroidism because it is more sensitive and less specific than TT4, causing more false-positive results.31,34
Treatment options include medical management with thioureylene antithyroid drugs (eg, methimazole), low iodine diet, radioactive iodine (I-131) therapy, and thyroidectomy.35 Methimazole (2.5 mg/cat PO every 12 hours) is most frequently used in the United States.35 Serum TT4 is measured 4 weeks later and the dose is gradually increased or reduced based on the results of repeated TT4 testing.35 Adverse effects include GI effects (eg, vomiting, anorexia), nonspecific signs (eg, depression, lethargy), blood dyscrasias (eg, thrombocytopenia, leukopenia, eosinophilia, lymphocytosis), pruritus and facial excoriations, and hepatopathy (rare).35 It is recommended that medical management with methimazole be attempted prior to more advanced treatment in order to screen for underlying CKD, which can be masked by hyperthyroidism secondary to increased glomerular filtration rate and decreased serum creatinine concentration.35
I-131 is the most effective option and the treatment of choice; it is typically safe and simple to administer but only available at specialized licensed facilities.35 I-131 is concentrated in the thyroid glands, where it emits β particles and γ radiation that destroy functional hyperplastic thyroid tissue.35 Thyroidectomy can be curative and may be performed when I-131 is not readily available.35 Anesthesia can cause the secondary metabolic, renal, and cardiac changes to elevate the American Society of Anesthesiologists status and increase the risk for perioperative complications. Thyroidectomy is required for patients with thyroid carcinoma (rare).35
4. Feline Myasthenia Gravis
Myasthenia gravis (MG) is a disorder of the neuromuscular junction and is either congenital or acquired. Congenital MG comprises a rare group of disorders characterized by failure of neuromuscular transmission due to inherited abnormalities of presynaptic, synaptic, or postsynaptic components.36 Acquired MG is an autoimmune disorder caused by antibodies (most often immunoglobulin G) directed primarily against nicotinic acetylcholine receptors (AChRs) on skeletal muscle.36 Functional loss of AChRs at the neuromuscular junction occurs via 3 possible mechanisms: complement-dependent lysis of the muscle membrane, cross-linking of AChRs leading to internalization of AChRs and decreased AChR half-life, and direct inhibition of AChR function by autoantibodies.36 A small percentage of patients have antibodies against other muscle proteins (eg, muscle-specific receptor tyrosine kinase, titin, ryanodine receptor). Methimazole is a potential cause of drug-induced MG in cats.37 Coexisting polymyositis has also been reported in cats.36
Abyssinian and Somali cats have a higher incidence of MG as compared with other breeds and crossbreed cats.37 Studies have found no sex predisposition. In a study, the average age of onset of clinical signs in 235 cats was 8.6 years (range, 6 months-15.4 years).38 Clinical signs of MG in cats are similar to those in dogs and include generalized weakness without regurgitation or dysphagia (50%), generalized weakness with regurgitation or dysphagia (40%), and focal MG with regurgitation or dysphagia without generalized weakness (10%).38 Facial weakness appears to be more common in cats.38 Megaesophagus and regurgitation are less common in cats because their entire esophagus is composed of smooth muscle. Cervical flexion has been reported in 20% of cats with acquired MG.39
Routine laboratory test (eg, CBC, serum chemistry profile, urinalysis) results are usually normal. Cats with MG are more likely to have a thymoma than dogs.38,40 A study identified a cranial mediastinal mass in 52% of cats with acquired MG that underwent thoracic radiography.38 A thymoma was identified in 97.7% of myasthenic cats that had a cranial mediastinal mass and underwent fine-needle aspiration.38 A large study of dogs with acquired MG identified thymoma in only 3.4% of affected dogs.40
The AChR antibody titer test, which is sensitive and specific, is considered to be the gold standard for diagnosing acquired MG.36 An edrophonium challenge test can be used as a screening tool; however, it lacks sensitivity and specificity, and edrophonium is no longer produced. A neostigmine challenge (40 µg/kg IM or 20 µg/kg IV) can be performed and the patient observed for improved strength.36 Electrodiagnostic testing (ie, repetitive nerve stimulation or single-fiber electromyography) or, in rare cases, immunohistochemical evaluation of junctional AChRs in whole-muscle biopsy specimens may be necessary to definitively diagnose acquired MG.36
Treatment in cats involves pyridostigmine ± immunosuppressive therapy. Cats seem to be more responsive than dogs to corticosteroids, which may be the only treatment needed.36 Thymectomy is recommended for cats that do not have evidence of local invasion or metastasis. Surgery may enable long-term survival and potentially be curative, but recurrence is possible.41
The prognosis for myasthenic cats appears to be worse than for dogs; 58% of cats in a study were euthanized because of the disease.38 In cats, there is a negative correlation between AChR antibody titer levels and survival; thus, cats with higher titers have a worse prognosis.38
ADDITIONAL DIFFERENTIAL DIAGNOSES FOR PASSIVE CERVICAL FLEXION IN CATS
Cervical fibrocartilaginous embolic myelopathy (presumably due to cervical muscular weakness associated with lower motor neuron dysfunction)
Diabetes mellitus
Organophosphate toxicity
Hereditary myopathy (particularly in Burmese and Devon rex cats)
Hypocalcemia
Hepatic encephalopathy
Polyneuropathy
Hypernatremic polymyopathy
Ammonium chloride toxicity
5. Idiopathic Polymyositis
Idiopathic polymyositis is a noninfectious, presumably immune-mediated, inflammatory myopathy in dogs and cats that can manifest as a generalized disorder or affect smaller muscle groups (eg, laryngeal muscles, extraocular muscles).42 Etiology has not been determined.
Although idiopathic polymyositis can occur in dogs and cats of all ages and breeds, predisposition has been reported in large-breed, adult, and older dogs.42-44 Clinical signs include exercise intolerance, stiffness, short-strided gait in all 4 limbs with a tiptoe appearance (ie, walking on eggshells), and lordosis.42 Involvement of the laryngeal muscles may cause dysphagia, dysphonia, or stridor.42 Cervical flexion is uncommon and may be passive due to neuromuscular weakness or active due to muscle pain.42
Although there are strict criteria for diagnosing idiopathic polymyositis in humans, these have not been defined in veterinary medicine. However, meeting at least 3 of the following criteria may strongly support a diagnosis42:
Appropriate clinical signs
Elevated serum CK
Abnormal electromyography with normal motor nerve conduction
Negative infectious disease and autoimmune titers
Inflammatory muscle biopsy
Serum chemistry profiles frequently demonstrate moderate to marked CK elevation and elevated ALT.43,45 Thoracic radiography is recommended to screen for megaesophagus even if there is no clinical evidence of regurgitation.42 Electromyographs often appear abnormal (eg, fibrillations, positive sharp waves, complex repetitive discharges), especially in the proximal appendicular muscles.42,45 Muscle biopsy of 2 muscle bellies is recommended. Histologic evaluation typically reveals mononuclear inflammation, necrotic muscle fibers with cellular infiltrates, and myofiber necrosis.45
Treatment consists of immunosuppression, pain relief, and supportive care for megaesophagus and aspiration pneumonia, if present. Immunosuppressive doses of corticosteroids are standard (ie, prednisone [dogs]/prednisolone [cats], 2 mg/kg PO every 12 hours for 2-3 days, then 1 mg/kg PO every 12 hours for 2 weeks)42 and should be gradually tapered to the lowest effective dose over several months based on response to treatment. Other immunosuppressants (eg, cyclosporine, azathioprine [dogs only]) may be required if clinical signs do not resolve or if adverse effects are intolerable to the patient or owner. Prognosis is good for patients without megaesophagus. Long-term, possibly lifelong, treatment may be necessary.42
Conclusion
Passive cervical flexion is a common abnormal body posture. The differential diagnosis list is relatively short, and diagnosis can typically be made easily. Passive cervical flexion must be differentiated from active cervical flexion because the differential diagnoses and neurologic examination findings are significantly different.