Top 5 Causes of Crusted Paw in Dogs
Alexander Werner Resnick, VMD, DACVD, Animal Dermatology Center
The distal extremity of the canine limb (ie, the paw) is composed of distinct anatomic structures and sites—the haired skin of the dorsal carpus and tarsus, the more glabrous palmar or plantar interdigital epidermis between the footpads, the footpads, the ungual or claw fold, and the claw—that each can suffer unique dermatoses. Dogs are often presented with concerns of a clinical “foot skin problem.” It is the clinician’s responsibility to determine which tissue(s) is affected and which disease(s) is the most likely cause.
A partial list of causes of crusted paw in the dog includes:
Infectious (bacterial, fungal, parasitic)
Allergic (atopy, food hypersensitivity, contact)
Immune-mediated (erythema multiforme, systemic lupus erythematosus, symmetrical lupoid onychodystrophy)
Endocrinopathy (hypothyroidism, hyperadrenocorticism)
Neoplastic (squamous cell carcinoma, melanoma, papilloma)
Interdigital cysts or granulomas1
The following reviews the more common causes of crusted paw from each category of disease.
1. Genetic: Digital Hyperkeratosis
Often termed nasodigital hyperkeratosis, this idiopathic disorder affects the footpads and nasal planum. Seborrheic breeds (eg, cocker and English springer spaniels, beagles, basset hounds, English bulldogs) are commonly affected.2,3 This is not primarily a crusting disease; accumulations of excessive keratinaceous debris develop on the entire surface of the footpads, with most being worn off of weight-bearing areas, leaving frond-like proliferations of keratin at footpad margins (Figure 1) and occasional crusting if fissuring occurs. Clinical signs typically develop in middle-aged to older dogs. Lesions are usually subclinical, but fissuring of the hyperkeratotic epidermis can cause pain and secondary infection.

Frond-like proliferations of keratin in a 15-year-old spayed spaniel crossbreed with digital hyperkeratosis.
Diagnosis is typically based on clinical signs and absence of lesions in the haired skin. Biopsy findings include epidermal acanthosis and marked orthokeratotic hyperkeratosis. Treatment focuses on relief of signs; the underlying cause cannot be resolved. Manual removal of excessive keratin is completed via trimming; frequent hydration to soften crusts and application of antiseborrheic compounds help improve patient comfort.
2. Metabolic: Superficial Necrolytic Dermatitis
Also referred to as necrolytic migratory erythema, hepatocutaneous syndrome, and metabolic epidermal necrosis, superficial necrolytic dermatitis (SND) is most often caused by hepatic disease and more rarely by a glucagon-secreting pancreatic tumor.4,5 Dermatitis has been associated with hypoaminoacidemia and/or other nutritional imbalances of the skin resulting in epidermal cell starvation.4,5
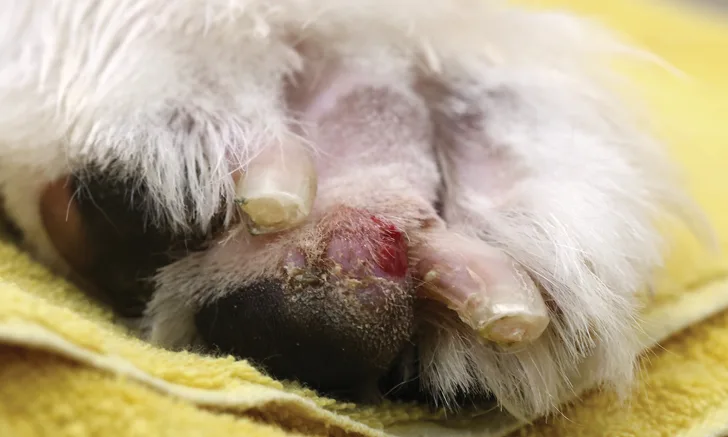
Exudative crusts with erythema in a 12-year-old neutered male terrier crossbreed with superficial necrolytic dermatitis.
SND affects older dogs. No breed predisposition is reported, but most cases seen by the author have been in smaller dogs. Lesions occur as thick accumulations of crust on pressure points (eg, elbows, footpads; Figure 2) and on the muzzle and periocular regions, often leading to erosions and ulcerations. The pinnae and genital regions are often affected by the time of presentation. Routine blood work may reveal nonregenerative anemia and often elevations in liver enzymes. Elevation in bile acids, decreased serum albumin, and hyperglycemia commonly develop. In the author’s experience, the development of diabetes mellitus is a poor prognostic indicator. A classic honeycomb appearance is often seen in the liver on abdominal ultrasound.6 Histologically distinct changes of parakeratotic hyperkeratosis and upper-level epidermal edema produce a red, white, and blue pattern. Secondary bacterial and/or yeast infections are common.
Diagnosis of SND is a marker of severe hepatic or metabolic disorder (or, rarely, glucagonoma). Prognosis is poor, with median survival time after diagnosis being 3 months.7 Nutritional support and amino acid IV infusions can prolong periods of comfort.
3. Parasitic: Demodectic Pododermatitis
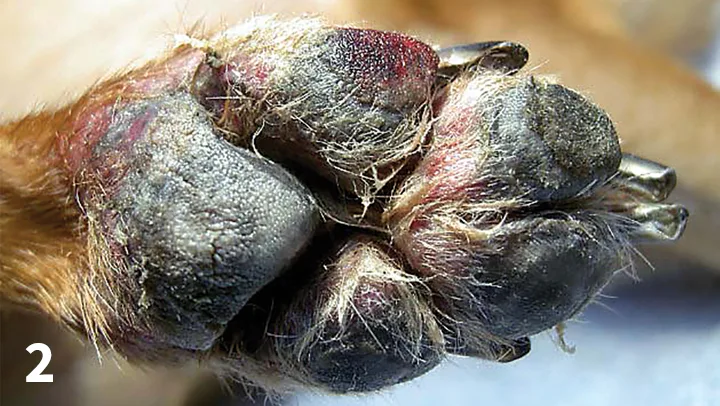
Demodicosis is an inflammatory disease caused by the proliferation of Demodex spp mites in hair follicles.8 This disorder is common in dogs younger than 1 year of age and uncommon in older dogs. Hereditary, treatment-related, and immunologic changes (often in combination) are suspected causes.8 Demodicosis most often presents with multiple alopecic lesions (Figure 3), which are more common in adult dogs and may be limited to the paws in patients without a history of generalized disease. Demodectic pododermatitis is associated with painful and deep secondary bacterial infection. The skin of all paws becomes alopecic, thickened, lichenified, exudative, and crusted. Footpads and claws are not affected, although the ungual folds may become swollen and exudative.

Demodectic pododermatitis in a 12-year-old neutered male terrier. Note the accumulations of crusts and alopecia affecting the skin of the carpus; the nails appear normal.
Diagnosis of demodectic pododermatitis can be challenging, as mites may be deep in follicles and difficult to find on scrapings; biopsies are occasionally needed. As with all cases of adult-onset demodicosis, an underlying cause should be determined. Treatment is identical to that for generalized demodicosis (eg, administration of antibiotics until secondary pyoderma has healed, topical antiseptic therapy, systemic parasiticides). Demodectic pododermatitis can be persistent and more difficult to treat than other forms of demodicosis.
4. Neoplastic: Mycosis Fungoides (Cutaneous Epitheliotropic Lymphoma)
The common name for cutaneous epitheliotropic lymphoma, mycosis fungoides (MF) is a rare disorder of older dogs, representing <1% of canine skin tumors.2 In MF, neoplastic T-cells with skin and adnexal structure tropism infiltrate the epidermis. Chronic inflammation, in particular chronic dermatitis (including allergic), may be a predisposing factor.2,9 Lesions are rarely confined to the paws but, when noted, are most often seen as depigmented and erythematous patches on the footpads (Figure 4) leading to ulceration with progression of disease. Footpad margins may be hyperkeratotic.
Diagnosis requires histopathologic examination of biopsy tissues, which will show a dense infiltrate of cytologically diverse lymphocytic cells in the epidermis and adnexal structures.2 Prognosis is poor, with a mean survival interval of 6 months to 2 years after diagnosis.9 Referral to an oncologist or dermatologist is recommended in suspected cases or following diagnosis.
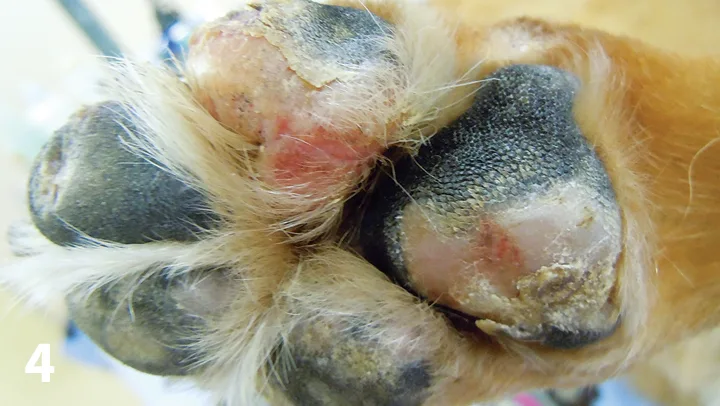
Depigmented and eroded patches on the footpads of a 12-year-old neutered male Akita with mycosis fungoides.
5. Immune-Mediated: Pemphigus Foliaceus
Pemphigus foliaceus (PF) is the most common autoimmune dermatosis of dogs.10 The primary lesion is a large, superficial pustule that rapidly ruptures, forming crusts with underlying erosions. Footpad margins are often affected, with adherent crusts (distinguishable from frond-like accumulations seen in nasodigital hyperkeratosis; Figure 5). Antikeratinocyte antibodies, specifically anti-desmoglein 1, have been identified in cases of canine pemphigus foliaceus, producing loss of intercellular adhesion in the upper layers of the stratum corneum (ie, acantholysis).10 Many factors, including genetics and drugs, are considered to be triggers for the induction of anti-keratinocyte antibodies.11
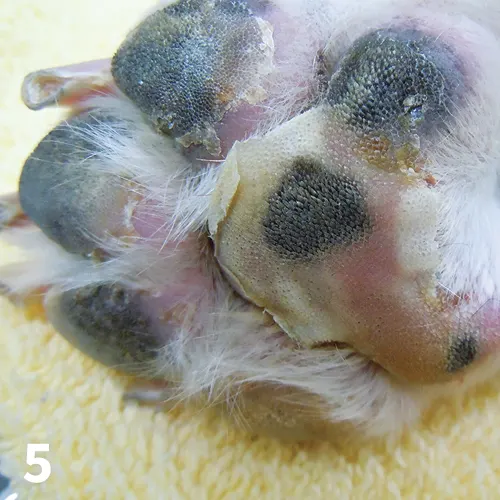
Accumulations of crusts at the margins of the footpads in a 2-year-old spayed American bulldog with pemphigus foliaceus. Note the peeling of epithelium from the pustule edges.
Although a tentative diagnosis may be made by finding acantholytic keratinocytes within pustule contents, definitive diagnosis requires histopathologic examination of biopsy tissues revealing subcorneal pustules with acantholytic cells, as well as intact neutrophils and scattered eosinophils.
Treatment requires immunosuppressive therapy. Prognosis is fair-to-good; cases benefit most with aggressive treatment to induce rapid remission, followed by reduced therapy to control disease.11
Conclusion
Changes in the paw secondary to trauma (self-induced or environmental) may obscure easy recognition of primary pathological processes. To assemble a list of differential diagnoses and pursue appropriate testing, practitioners should note how many paws are affected, which regions are affected, and whether lesions are present on other areas of the body.