Top 5 Breed-Specific Considerations to Avoid Adverse Drug Effects
Katrina L. Mealey, DVM, PhD, DACVIM, DACVCP, Washington State University
Michael H. Court, BVSc, PhD, DACVAA, Washington State University

Adverse drug effects can increase morbidity and mortality in dogs, cause emotional stress for pet owners, and increase cost of care. Many (but not all) adverse drug effects can be predicted and therefore prevented. Genetic testing can be used to help identify possible drug effects and determine whether dose adjustments or alternative drug therapies are needed.
Following are the top 5 adverse drug effects that are more likely to occur in specific dog breeds, according to the author.
1. MDR1 Mutation & Enhanced Susceptibility to CNS Toxicity (Primarily in Herding Breeds)
P-glycoprotein, encoded by the multidrug sensitivity gene (MDR1 gene, also known as ABCB1 gene), functions as a drug transport pump at the blood-brain barrier, preventing potentially toxic compounds from gaining access to the brain.1 The MDR1 gene mutation (ABCB1-∆) results in production of dysfunctional P-glycoprotein and affects many herding breed dogs (Table 1) and some nonherding breeds.2 Drugs that are P-glycoprotein substrates achieve higher brain concentrations in dogs with the MDR1 mutation (heterozygous or homozygous) than in dogs without the mutation.1 When P-glycoprotein substrate drugs exert CNS effects, those effects are more pronounced in dogs with the MDR1 mutation unless the dosage is decreased appropriately.1,3 Thus, dose reductions should be made when possible or an alternative drug should be selected. A nuclear scintigraphy study demonstrated that wild-type MDR1 homozygotes (MDR1 normal/normal) have a fully functional blood-brain barrier with essentially no radioactivity in the brain, whereas MDR1 mutant homozygotes (MDR1 mutant/mutant) have brain radioactivity levels comparable with surrounding tissue, demonstrating a dysfunctional blood-brain barrier with respect to P-glycoprotein substrates (Figure 1).1 Although many P-glycoprotein substrate drugs (Table 2) exert CNS effects and cause neurologic toxicity in dogs with the MDR1 mutation, some do not and can therefore be administered at usual dosages. MDR1 genotyping should be performed to identify at-risk dogs prior to treatment with P-glycoprotein substrate drugs.4
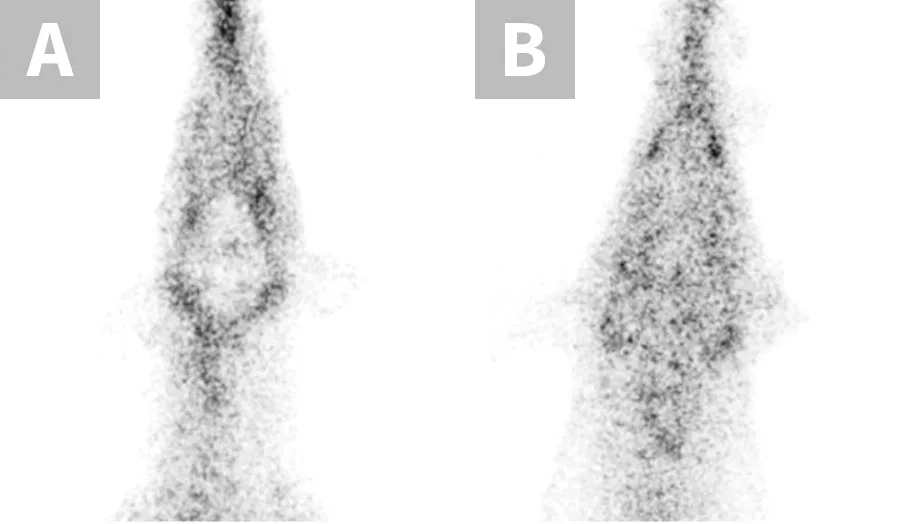
Nuclear scintigraphic images of the brain and surrounding tissue in collies after IV injection of the radiolabeled P-glycoprotein substrate sestamibi (99mTc-sestamibi). P-glycoprotein restricts 99mTc-sestamibi entry into brain tissue of the MDR1 wild-type dog (A), whereas lack of P-glycoprotein allows 99mTc-sestamibi to enter brain tissue of the MDR1 mutant/mutant dog (B).
Table 1: Dog Breeds Known to Carry The MDR1 Mutation24-26
*Formerly longhaired whippet
Table 2: Drugs & Their Potential Adverse Effects in Dogs with the MDR1 Mutation1-3,12,13
*Should only be administered at label doses; label doses for heartworm prevention undergo safety studies in dogs with the MDR1 mutation as required by the FDA.
2. Deficiency in Greyhounds & Other Sighthounds
Greyhounds recover more slowly than other dog breeds after receiving certain injectable anesthetic drugs (eg, thiopental, thiamylal, propofol).5,6 Accumulating evidence suggests this is largely due to decreased liver expression of cytochrome P450 2B11 (CYP2B11; a major drug-metabolizing enzyme) in affected dogs.7 CYPB11 metabolizes a range of anesthetic drugs, including propofol, ketamine, midazolam, and medetomidine.7-9 A mutation in the CYP2B11 gene (CYP2B11-H3) that decreases CYP2B11 expression in vitro was recently identified in greyhounds and certain other sighthound breeds.10 The mutation has higher prevalence in American Kennel Club-registered greyhounds than in National Greyhound Association-registered greyhounds.10 This difference may be a consequence of selective breeding for different purposes (ie, conformation vs racing speed). CYP2B11-H3 was also identified in >50% of the sighthound breeds that were evaluated, as well as in some nonsighthound breeds at a lower prevalence (Table 3).10 Although in vivo validation studies are still needed, CYP2B11 genotyping might aid in identification of individual dogs likely to demonstrate prolonged effects when receiving drugs that require the CYP2B11 enzyme for efficient elimination.
Table 3: Dog Breeds Known to Harbor the CYP2B11-H3 Mutation
*Formerly longhaired whippet
3. MDR1 Mutation & Enhanced Susceptibility to Drugs Eliminated via Biliary Excretion (Chemotherapeutics & Other Drugs)
Adverse drug effects caused by the MDR1 gene mutation are not limited to neurologic toxicity. Because P-glycoprotein actively transports substrate drugs into the bile, dogs with the MDR1 mutation have decreased biliary clearance of those drugs normally eliminated via biliary excretion (Figure 2), resulting in increased overall drug exposure.11 Affected dogs experience susceptibility to associated adverse effects when the drugs are administered at recommended dosages. This effect has been documented with vincristine (bone marrow suppression),12 cyclosporine A (immunosuppression),13 doxorubicin (bone marrow suppression, GI toxicity; anecdotal), and others. Affected dogs should receive decreased dosages of these drugs as previously described.14 MDR1 genotyping should be performed to identify at-risk dogs prior to treatment with P-glycoprotein substrate drugs.4
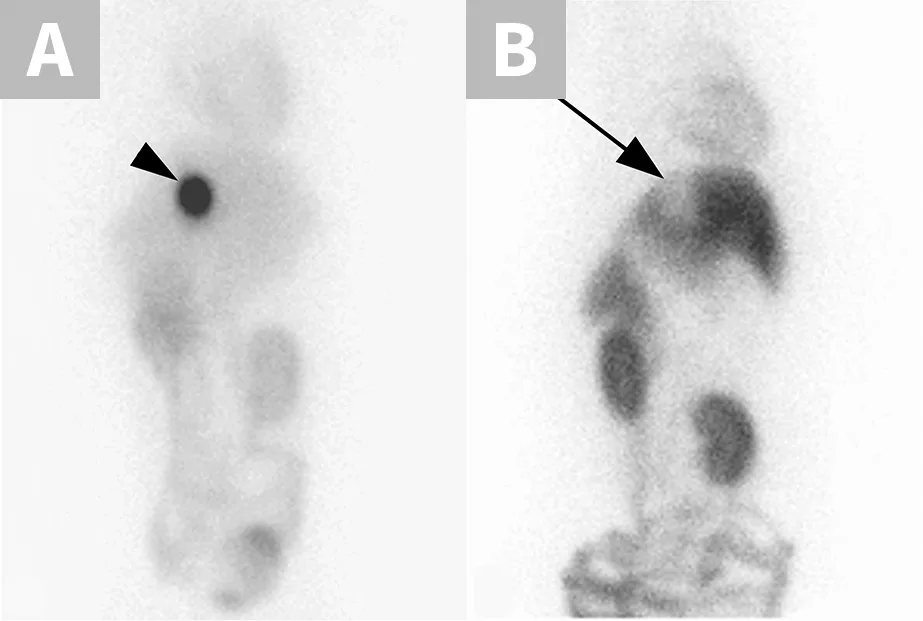
Nuclear scintigraphic images of the ventral abdomen of an MDR1 wild-type dog (A) and an MDR1 mutant/mutant dog (B) 2 hours after IV injection of the radiolabeled P-glycoprotein substrate sestamibi (99mTc-sestamibi). P-glycoprotein efficiently pumps 99mTc-sestamibi into the gallbladder in the MDR1 wild-type dog (arrowhead). In stark contrast, biliary excretion is essentially nonexistent in the MDR1 mutant/mutant dog (arrow).
4. Delayed Postoperative Bleeding in Greyhounds & Other Sighthounds
Significant and occasionally life-threatening postoperative bleeding that starts 24 to 48 hours following surgery has been identified as a significant health concern in greyhounds.15 Clinical studies suggest the incidence of delayed bleeding can range from 26% following routine gonadectomy16 to ≤67% following limb amputation for osteosarcoma.17 Current evidence indicates reduced α2-antiplasmin activity in the plasma of affected dogs, suggesting that bleeding may be secondary to enhanced fibrinolysis of newly formed clots.18,19 Both retrospective and placebo-controlled prospective studies have established the effectiveness of treatment with epsilon aminocaproic acid (EACA; Table 4), an antifibrinolytic drug, for preventing delayed bleeding via increased clot strength.16,17 Anecdotal evidence suggests Scottish deerhounds are also susceptible to delayed postoperative bleeding and may benefit from preventive antifibrinolytic treatment (EACA or tranexamic acid).20 A breed-based predisposition to this condition has not yet been reported, but it is likely caused by a mutation in a gene that regulates fibrinolysis. Although current recommendations are to treat all affected breed dogs, genetic testing for the putative mutation could be used to identify individual dogs that would benefit from prophylactic antifibrinolytic treatment. Identifying dogs of affected breeds that do not require treatment (ie, those that lack the mutation) could also minimize the potential risk for adverse effects of antifibrinolytic drugs (eg, thromboembolism).
Table 4: EACA Dose Rates Currently Use for the Prevention of Delayed Postoperative Blooding in Greyhounds27 and Scottish Deerhounds20
*EACA tablets are administered PO every 8 hours for 5 days starting on the day of surgery.
5. Sulfonamide Hypersensitivity in Doberman Pinschers
Hypersensitivity to sulfonamides can manifest as fever, keratoconjunctivitis sicca, hepatotoxicity, skin eruptions, blood dyscrasias, and/or arthropathy and may lead to a mortality rate of 21% in Doberman pinschers.21 Doberman pinschers have been reported to be particularly predisposed to sulfonamide hypersensitivity, but this is not limited to this breed.22 A recent study identified an association between a mutation in the cytochrome b5 reductase (CYB5R3) gene and sulfonamide hypersensitivity.23 This mutation was determined to be overrepresented in Doberman pinschers and in dogs of other breeds that experienced sulfonamide hypersensitivity reactions. Although CYB5R3 encodes a drug-metabolizing enzyme, this enzyme does not appear to be directly involved in the metabolism of sulfonamides.23 Instead, it is likely to be linked to a polymorphism that is directly involved in sulfonamide clearance. When possible, sulfonamides should be avoided in Doberman pinschers.
Conclusion
The physical characteristics of certain dog breeds can provide clues for breed-specific susceptibility to certain adverse drug reactions. Genotyping for specific variants can be used to inform appropriate drug selection and/or dosage modifications. Preventive treatment with EACA or tranexamic acid should be considered in greyhounds and Scottish deerhounds undergoing major surgery after assessment of the risks and benefits to individual dogs.