Recurring Pruritic Skin Lesions in an English Bulldog
Ashley E. Detwiler, DVM, DACVD, MedVet, Cincinnati, Ohio

Hope, a 15-month-old, 39.6-lb (18-kg) intact female English bulldog, is presented to a dermatologist for recurring diffuse skin lesions.
History
On initial presentation to the primary clinician, Hope had generalized scaling, crusts, and pustules, as well as a recent history of whelping. The owner requested euthanasia, but Hope was instead relinquished and adopted.
Bilateral third eyelid prolapse, keratoconjunctivitis sicca, and a grade II/VI heart murmur with a left-sided point of maximum intensity were diagnosed. Skin scrapings were negative, aerobic bacterial culture and susceptibility testing isolated methicillin-resistant Staphylococcus pseudintermedius, and skin biopsy results were consistent with secondary bacterial pyoderma and suspected allergic hypersensitivity.
Skin lesions resolved and hair regrew following transition to a commercial hydrolyzed protein diet, administration of marbofloxacin based on bacterial culture and susceptibility testing, and bathing with chlorhexidine and dilute bleach every 48 hours.
Diffuse lesions recurred and pruritus was rated as 10/10 on the pruritus visual analog scale 1 to 2 weeks after marbofloxacin was discontinued. Methylprednisolone (0.4 mg/kg PO every 24 hours, divided and given every 12 hours) was administered. Estrus occurred ≈2 weeks after lesions recurred, and referral to a dermatologist was made.
Presentation
On presentation to the dermatology clinic, Hope is no longer in estrus. Pruritus is described as 6/10 on the pruritus visual analog scale, despite strict feeding for 12 weeks of the commercial hydrolyzed protein diet without additional food items or treats. She is not current on ectoparasite preventives.
Physical Examination
Dermatologic examination reveals diffuse pustules on the concave pinna and margins of both ears; otitis is not present in the ear canals. Severe and diffuse patchy erythema, pustules, papules, plaques, and excoriations are present along the head/muzzle, dorsal trunk, ventral neck, extremities, inguinal area, mammae/nipples, and perianal area (Figure 1). There is marked proliferation of the tissue with erythema of the palmar and plantar interdigital spaces and hyperkeratosis of the paw pads with loss of paw pad margins. The ventral neck and pelvic limbs have marked edema.
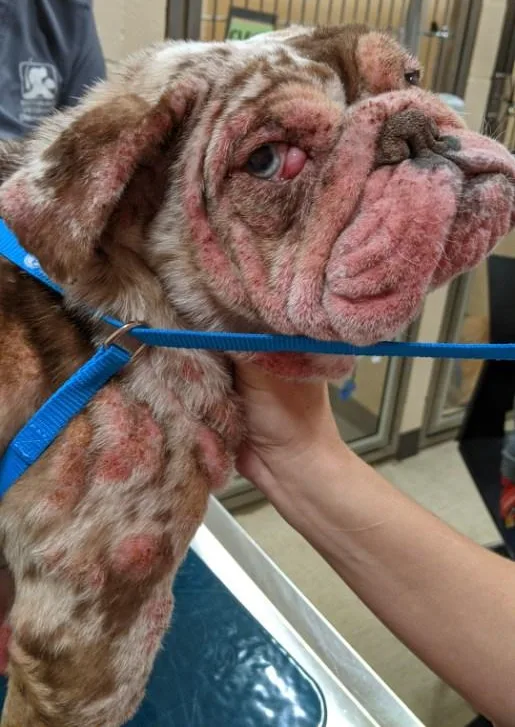
FIGURE 1A
Patchy alopecia, erythema, crusts, and swelling of the skin on the face, muzzle, margins of the pinnae, cranial trunk (A), and dorsal trunk (B) on initial presentation to the dermatology clinic can be seen. There is also marked proliferation of the tissue with erythema of the plantar interdigital spaces and digits and abnormal appearance of the footpads (C).
How would you diagnose and treat this patient?
Diagnosis
Skin lesions raised clinical suspicion for a condition other than allergic hypersensitivity with secondary bacterial pyoderma. Differential diagnoses included pemphigus foliaceus (an autoimmune skin disease), other immune-mediated skin diseases, cutaneous histiocytosis, and epitheliotropic lymphoma.
Cytologic examination of skin pustules revealed marked neutrophilic and eosinophilic inflammation, rare cocci, and occasional rounded keratinocytes consistent with acantholytic cells. Pemphigus foliaceus was suspected based on clinical and cytologic findings. A second biopsy was recommended because of nonspecific initial histopathology results.
Two weeks after initial presentation to the dermatology clinic, Hope was anesthetized with IV butorphanol and propofol, and biopsy was performed. A combined injection of articaine HCl and epinephrine was administered subcutaneously at biopsy sites for analgesia. An 8-mm punch was used to obtain biopsies from 5 representative sites on the neck, dorsum, and edematous lateral limbs; one biopsy was submitted for fungal and aerobic bacterial culture. Hyperkeratotic crusts from the paw pads were also submitted for evaluation. Biopsy sites healed without complication.
Histopathology of the neck, dorsum, and limb biopsies revealed severe, chronic, hyperplastic, subcorneal crusting–pustular dermatitis with acantholytic cells. The paw pad sample revealed crusting–pustular epidermitis with acantholytic cells, neutrophilic and eosinophilic exocytosis, and dermal edematous inflammatory cell infiltration. Results confirmed diagnosis of pemphigus foliaceus.
Treatment & Outcome
Treatment was initiated with anti-inflammatory doses of prednisone and modified cyclosporine to induce remission (see Pemphigus Foliaceus Medications for administration information). Clinical signs, particularly crusted lesions and pruritus, were difficult to control, likely due to concomitant allergic dermatitis.
PEMPHIGUS FOLIACEUS MEDICATIONS
Initial
Prednisone: 1.5 mg/kg PO every 24 hours for 7 days, tapered to 1.25 mg/kg PO every 24 hours for 7 days, then 0.5 mg/kg PO every 24 hours
Modified cyclosporine: 2.5 mg/kg PO every 24 hours for 14 days, increased to 5 mg/kg PO every 24 hours
8 weeks after initial
Prednisone: 0.5 mg/kg PO every 24 hours
Modified cyclosporine: reduced over 4 to 6 weeks with intention to discontinue
Azathioprine: 1.25 mg/kg PO every 24 hours for 14 days, tapered to 1.25 mg/kg PO every 48 hours
6 months after initial; spring/summer allergy seasons
Prednisone: 0.25 mg/kg PO every 24 hours
Azathioprine: 1.25 mg/kg PO every 48 hours
Triamcinolone 0.1% cream
Oclacitinib: 0.8 mg/kg PO every 12 hours for 14 days, tapered to 0.8 mg/kg PO every 24 hours
Long-term
Prednisone: 0.25 mg/kg PO every 24 hours
Azathioprine: 1.25 mg/kg PO every 48 hours
Oclacitinib (spring/summer allergy seasons only): 0.8 mg/kg PO every 12 hours for 14 days, tapered to 0.8 mg/kg PO every 24 hours
Because of adverse effects of prednisone and lack of clinical response after ≈8 weeks of treatment with prednisone and cyclosporine, azathioprine was added as a third immunomodulatory medication with monitoring for GI upset and clinical pathology changes (eg, hepatotoxicity leading to liver enzyme elevations, bone marrow suppression causing reduced WBC and RBC indexes). Cyclosporine was reduced over the next 4 to 6 weeks until it could be discontinued.
Pruritus worsened 6 months after initial presentation to the dermatology clinic following reduction of cyclosporine and nearly complete remission of skin lesions, possibly due to spring tree and grass pollen. Triamcinolone 0.1% cream was applied to affected areas, and oclacitinib was administered as adjunctive antipruritic therapy in conjunction with azathioprine and further tapering of prednisone.
CBC and serum chemistry profile were monitored every 2 weeks by the primary clinician to evaluate liver enzymes and bone marrow responses. Hope was spayed ≈6 months after diagnosis; at that time, skin lesions were markedly improved, hair coat had regrown with good quality, and overall behavior, energy, and activity were improved (Figure 2).
Long-term therapies include prednisone (subtherapeutic dose) and azathioprine, as well as oclacitinib during the spring and summer allergy seasons. There is limited information on interactions and adverse effects of coadministration of oclacitinib, prednisone, and azathioprine; this combination is administered out of necessity following a discussion with the owner regarding quality of life and balance of adverse effects with continued remission of autoimmune and allergic skin disease. Hope is evaluated by the dermatologist annually. Depending on long-term follow-up examination results, therapies may be permanently required.
Listen to the Podcast
Dr. Detwiler dives deeper into pemphigus foliaceus in this episode of Clinician's Brief: The Podcast.