Quiz: Nutritional Support in a Puppy with Parvoviral Enteritis
Daniel L. Chan, DVM, DACVECC, DECVECC, DACVIM (Nutrition), DACVN, MRCVS, University of London, North Mymms, Hertfordshire, United Kingdom
Eleanor Haskey, BSc(hons), Royal Veterinary College, University of London
In critically ill puppies, placement of nasogastric feeding tubes can be an effective means for providing nutritional support.
A 9-week-old, 6.8-lb (3.1-kg) male border terrier was presented for collapse and a 4-day duration of vomiting, and diarrhea. The puppy, acquired from a breeder, had been in the owner’s possession only 6 days, was the only pet in the household, and had never been outdoors. He had been treated for intestinal parasites and fleas, had been microchipped, and had received an initial vaccine (including canine parvoviral antigen) before being acquired by the owner.
Physical Examination
On presentation, the patient was laterally recumbent and minimally responsive. Heart rate was 80 bpm with no murmur, peripheral pulse quality was poor, respiratory rate was 24 breaths/min with audible lung sounds bilaterally, and rectal temperature was 97.7°F (36.5°C). Noninvasive blood pressure (via Doppler) was 90 mm Hg. The abdomen was distended and slightly painful on palpation but no obvious intussusception was palpated, and the patient was estimated to be moderately dehydrated.
Diagnostic Results
An over-the-needle jugular catheter was placed and blood was collected for an emergency database and venous blood gas. Results revealed hypokalemia (2.6 mEq/L; reference interval, 3.6-4.6 mEq/L), hypoglycemia (64.8 mg/dL; reference interval, 88-130 mg/dL), mild anemia (PCV 21%; reference interval, 29%-35%), and hypoproteinemia (total solids, 4.5 g/dL; reference interval 5.0-6.0 g/dL). Blood smear evaluation was suggestive of leukopenia. A 0.5 g/kg dextrose bolus IV was adminstered, followed by a 20 mL/kg IV bolus of a balanced isotonic crystalloid fluid. A fecal parvovirus antigen test confirmed parvoviral enteritis. An abdominal ultrasound was performed and findings were suggestive of enteritis (Figure 2).
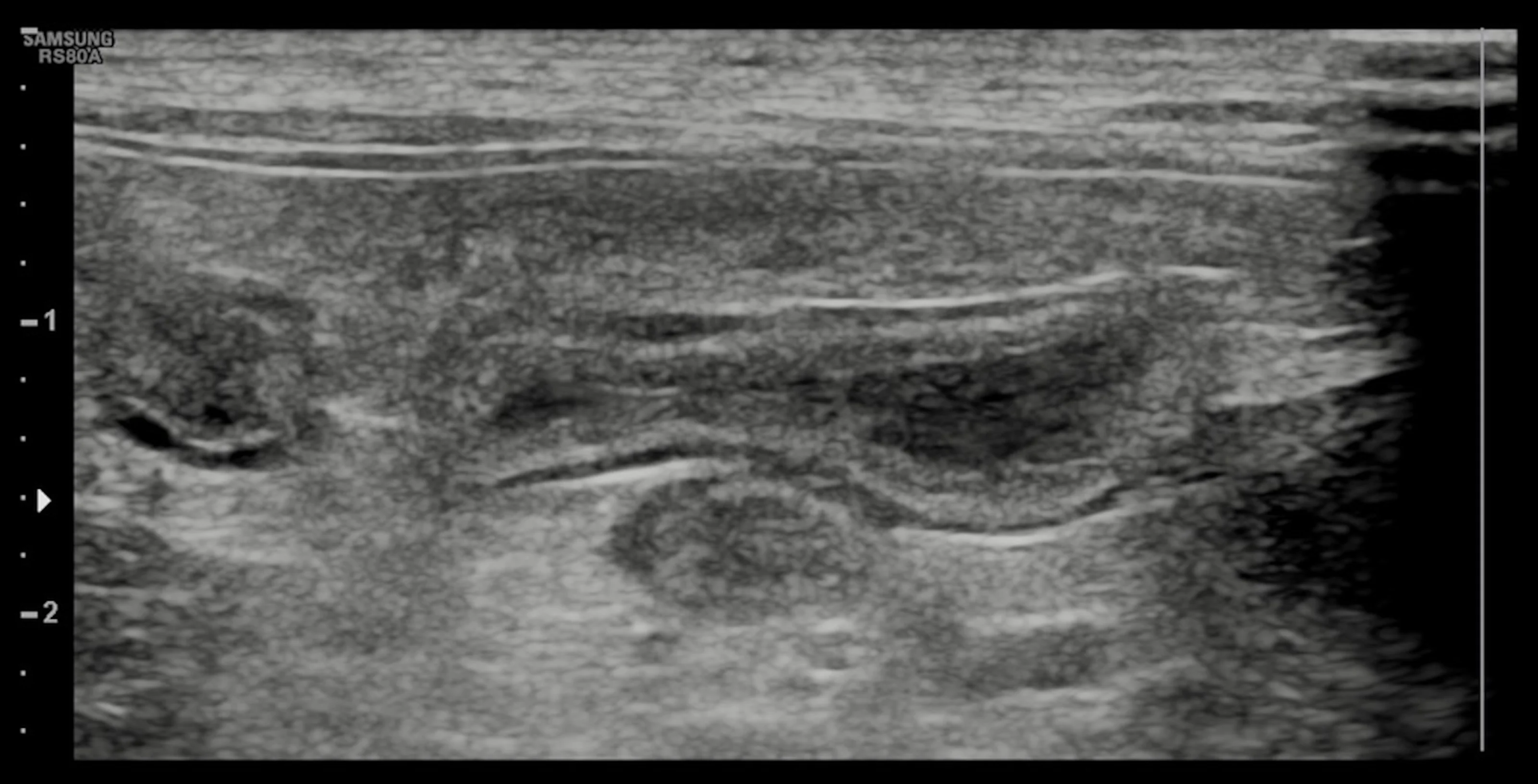
Abdominal ultrasound demonstrated no evidence of small intestinal obstruction or intussusception; findings were suggestive of enteritis.
Treatment
Following fluid resuscitation, the patient was placed in an incubator to help regulate body temperature, and strict barrier nursing protocols were implemented. To address ongoing fluid losses and dehydration, a balanced crystalloid fluid, supplemented with 5% dextrose and 40 mEq/L KCl, was administered at 6 mL/kg/hr (not exceeding the maximum safe dose of 0.5 mEq/kh/hr). Methadone (0.1 mg/kg IV q6h) was administered for analgesia. Metoclopramide (0.01 mg/kg/h IV CRI) and ondansetron (0.8 mg/kg IV q24h) were administered to manage nausea and vomiting. Ampicillin (22 mg/kg IV q8h) was administered because of the concern for development of bacterial sepsis.1
To address the patient’s nutritional needs, a 5-French nasogastric feeding tube was placed 12 hours after presentation. Enteral feeding, even in patients with active vomiting, is the preferred route of feeding. Only when vomiting is protracted and unresponsive to treatment would parenteral nutrition be considered.
There are numerous advantages associated with enteral feeding. For example, placing the tip of the feeding tube into the stomach allows for removal of fluid accumulating in the stomach, which contributes to regurgitation and patient discomfort. In addition, initiation of early enteral feeding has been shown to improve GI motility and reduce vomiting.2,8 In this case, antiemetics and suction of gastric residual volumes were used to further improve tolerance to enteral feedings. Gastric residual fluid removed ranged from 5 mL to 20 mL over 6 hours. Because gastric fluid was not returned to the patient, acid-base status and electrolytes were monitored.
An enteral liquid diet containing high-quality and highly digestible protein with moderate fat restriction (to aid in gastric emptying2-4) and reasonable caloric density (1 kcal/mL) was chosen. Enteral feedings were initiated at 50% of resting energy requirement (RER) for the first day of feeding and subsequently increased to 100% RER. RER for this patient was calculated at 163 kcal per day using the equation RER = 70 x (body weight)<sup0.75sup>. Because critically ill animals have energy requirements that approximate RER5,6 and the presence of GI dysfunction (eg, vomiting, regurgitation) decreases their tolerance to enteral feeding,2,7 the patient’s diet was gradually increased from 50% of RER to 100%. Feeding critically ill animals in excess of RER has also been shown to increase complications.6,7 The enteral diet was delivered as a continuous infusion using a syringe pump at 3 mL/hr on the first day and 6 mL/hr on subsequent days. As the enteral liquid diet provided additional hydration, the rate of IV fluids was reduced by 2 mL/kg/hr. As hypoglycemia and hypokalemia resolved, parenteral fluids were discontinued on day 3.
Over the next several days, the frequency of vomiting and diarrhea reduced. The patient began eating voluntarily, and tube feeding was discontinued. Methadone, metoclopramide, and ondansetron were also discontinued, and the patient was discharged on day 6.
GLOBAL RELEVANCE
Parvoviral enteritis is a common disease in young dogs, with high morbidity worldwide. Effective vaccination against the disease is available and can reduce the incidence of disease; nonetheless, without broader accessibility to vaccination many nonvaccinated dogs succumb to the disease. Affected dogs can have severe vomiting and diarrhea that may lead to shock, sepsis, and/or death. Treatment focuses on fluid therapy, cardiovascular support, management of nausea and vomiting, and antibiotics. Nutritional support plays a critical role in enhancing recovery and should form an integral part of the treatment plan.