Progressive Facial Lesion in a Community Cat
Sarah Steen, DVM, Critters Without Litters, Bakersfield, California
Lisa M. Pohlman, DVM, MS, DACVP, Kansas State University
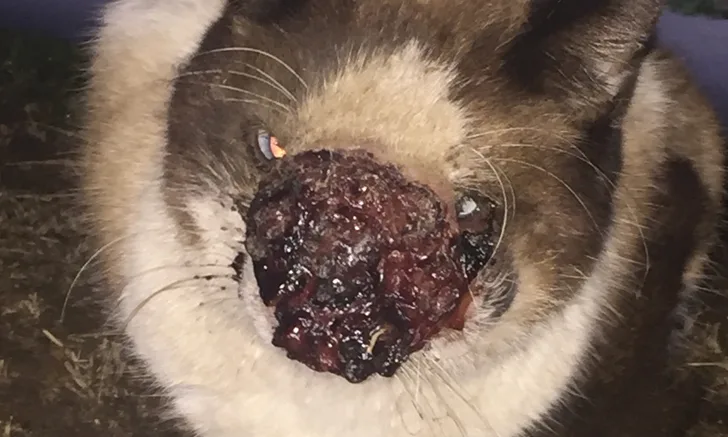
Nonose with a large, ulcerated, and encrusted lesion 35 days prior to trapping. He ate the provided food but would not enter the trap for food at the time this photograph was taken. Photo courtesy of Frankie Cowan
Nonose, an intact male community cat in Bakersfield, California, estimated to be 3 to 5 years of age, had a slowly progressive facial lesion and audible respiratory noise of ≈1 year’s duration, based on reports by his community caretakers. The facial lesion was described as alternating between a visibly encrusted (Figure 1) or an open bleeding wound (Figure 2). According to local community members, Nonose had been in the area for years; he had no known owner, and multiple caretakers provided him food but were never able to handle him. Nonose was trapped and transported to a local clinic to assess the nature and extent of his disease.
Physical Examination
On visual examination, Nonose was bright and alert, with severe respiratory stertor and subjectively increased inspiratory effort. No open-mouth breathing was noted during transport or at the clinic. He had a large lesion extending from the medial canthi across the forehead to just dorsal to the margin of the upper lips. Within these margins, no normal haired skin, nasal planum, or nares could be identified. He appeared to be visual, and mentation was deemed appropriate.
Diagnosis
Nonose was anesthetized for further examination. The surface of the lesion was characterized by a glistening, serosanguinous, gelatinous material (Figure 3), which, when wiped with gauze, revealed exposed bone or cartilage. The full-thickness lesion extended into the mouth and through the upper left lip, creating a hole into the oral cavity (Figure 2). The proliferative nature of the lesion likely obstructed vision directly in front of the patient. Nasal passages were located after debridement but were composed of exposed nasal bone/cartilage rather than planum. All upper incisors were absent, and the gingiva surrounding all remaining teeth and the hard palate appeared affected with diffuse erythema and small amounts of the gelatinous material (Figure 4).
Due to the severity of facial tissue destruction, poor prognosis, and feral nature of the cat, euthanasia was elected. A blood sample was obtained prior to euthanasia for point-of-care FeLV/FIV testing, which was performed immediately after euthanasia and was negative. Multiple impression smears of affected tissue were obtained for cytologic examination (Figure 5).
Smears were composed of abundant round to oval-shaped, blue to pink yeast structures 3 to 12 µm in diameter. A thick, clear capsule surrounded the structures, resulting in organisms ≈5 to 20 µm in diameter. Narrow-based budding of the organisms was observed. Among the organisms were large, often vacuolated macrophages that occasionally contained one or more of the yeast structures (Figure 5).

FIGURE 2
Nonose on arrival to the clinic immediately after being trapped. A hole extending into the oral cavity (arrow) is visible.
DIAGNOSIS:
CRYPTOCOCCOSIS
TREATMENT AT A GLANCE
Culture and susceptibility testing is recommended prior to initiating treatment and can help guide selection of appropriate antifungal therapy and provide prognostic information.2
Azoles are the treatment of choice, with fluconazole and itraconazole most commonly used in cats.3
Surgical excision and/or debulking may help decrease the required duration of antifungal therapy and increase the chance for infection resolution, although lesion location may hinder the ability of these to be performed.2
Treatment should be continued until clinical signs are no longer present and fungal antigen titers are 0.1,3
Antifungal therapy duration ranges from 2 to 18 months, with an average duration of 4 to 6 months.1,2
Discussion
Cryptococcal infections are seen worldwide in various species and, in the United States, are most common in California and the Pacific Northwest.1 Basidiospores are usually found in soil or avian fecal material; infection often occurs through inhalation but can occur via direct contact of basidiospores in open wounds.1-3 Incubation can range from a few months to years.2
Although assays to determine species were not performed in this patient, most cats in California that have cryptococcosis are infected by Cryptococcus gattii VGIII, with relatively fewer infections being due to C gattii VGII.1 C neoformans var grubii is the most common cause of cryptococcosis in dogs and humans; in the United States, cats are rarely infected with this species.1
In cats, cryptococcosis is generally chronic and often presents as mucosal lesions in the nasal cavity, regardless of the primary site of entry/infection of the basidiospores.1-3 The glistening, serosanguinous gelatinous nature of the mass observed in this patient is a characteristic feature of cryptococcosis and a reflection of the presence of the polysaccharide capsule.1,2 Meningoencephalitis, cerebral granulomas, chorioretinitis, optic neuritis, uveitis, and other lesions may also be observed.2,3
Pathogenesis of disease and success of treatment are dependent on the type and extent of infection, host immunity, and strain of Cryptococcus spp involved.1-3 Fungal culture is recommended, as a long course of therapy is required to resolve infection, and antifungal resistance is common.1-3 Antifungals commonly selected for feline therapy include fluconazole (10 mg/kg PO every 12 hours) and itraconazole (5-10 mg/kg PO every 24 hours). Fluconazole is the initial antifungal agent of choice due to its good tissue penetration in the brain, eyes, and urinary tract and its relatively low cost. If the patient fails to respond to fluconazole therapy, as is often seen with C gattii infections, itraconazole may help achieve remission; however, multimodal therapy, including amphotericin B and 5-flucytosine, may be required in severe disseminated cases. Serial laboratory monitoring of liver enzymes is recommended, as liver toxicity is possible with azole therapy (see Treatment at a Glance).1-3
Once cryptococcosis is diagnosed, a discussion should be held with the owner regarding the cost of long-term medication and laboratory monitoring, the importance of owner and patient compliance for long-term oral therapy, and the potential for disease recurrence, particularly if compliance is poor. A committed owner and a compliant patient are essential for a successful outcome.
Treatment success can be gauged by reduction in both clinical signs and serum antigen titers (at least one dilution per month of treatment); treatment should be continued until the antigen titer is 0.1-3 Continued antigen titer monitoring after resolution of disease at 3- to 6-month intervals is recommended, as early detection of relapse can lead to shorter duration of repeat treatment (see Take-Home Messages).2
TAKE-HOME MESSAGES
In the clinical setting, cytology is often used to make an initial diagnosis of cryptococcosis.1-3
Although cryptococcosis in humans is more common in immunocompromised patients, FeLV/FIV status in cats does not appear to play a role in susceptibility to Cryptococcus spp. However, coinfection with FIV/FeLV may impact response to therapy and patient prognosis.1-3
Cryptococcosis can develop after inhalation of basidiospores from the environment2,3; infected patients are considered noncontagious.
Young adult cats appear to be at increased risk for infection, with the median age of infected cats being 6 years1; cats of all ages may be affected.
Treatment can be successful, but owner and patient compliance, as well as duration of therapy and resulting financial requirements, may inhibit success.1
With appropriate treatment, nasal and cutaneous diseases may have a good prognosis. If CNS or ocular disease is present, treatment is generally less effective.4