Polyuria & Polydipsia in a Dog
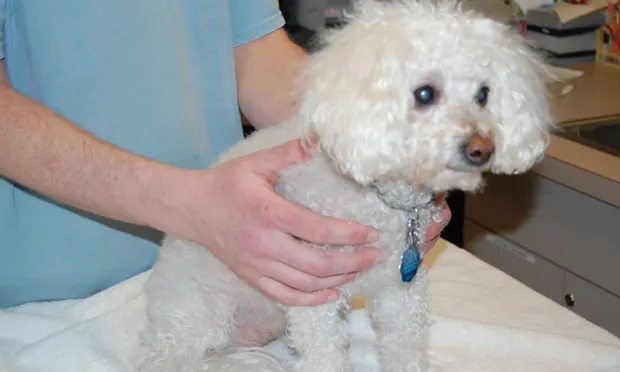
A 10-year-old, 6.8-kg spayed bichon frise was presented for a 2-month history of progressive polyuria and polydipsia and 5-month history of progressive alopecia with intermittent superficial pyoderma.
HistoryThe owner reported that the dog had no vomiting or diarrhea, but its appetite had increased. At routine evaluation 6 months before presentation, the dog weighed 6.3 kg. It was not receiving any medication.
Physical ExaminationThe dog was bright and alert. Vital signs were within normal limits. Physical examination revealed marked truncal alopecia but no evidence of superficial pyoderma (Figure 1). The abdomen appeared rounded; mild cranial organomegaly was present.


Figure 1. On examination, the patient showed marked truncal alopecia (A) without any evidence of superficial pyoderma (B).
Diagnostics & ImagingLaboratory studies were conducted and evaluated (Table). Adrenocorticotropic hormone (ACTH) stimulation testing showed a baseline cortisol concentration of 4.5 µg/dL (range, 2–6 mg/dL) and post-ACTH cortisol concentration of 9.6 µg/dL (range, 6–18 mg/dL). Low-dose dexamethasone suppression testing revealed a baseline cortisol concentration of 4.5 µg/dL, 4-hour cortisol concentration of 4.3 µg/dL, and 8-hour cortisol concentration of 4.3 µg/dL (range, <1 µg/dL = normal at 4 and 8 hr; 1–1.4 µg/dL = inconclusive; ≥1.5 µg/dL = positive).
Figure 2. Abdominal ultrasound showing enlargement of the left adrenal gland with a 1.19-cm nodule (arrow) on the cranial pole
Abdominal ultrasonography showed enlargement of the left adrenal gland with a 1.19-cm nodule on the cranial pole (Figure 2). The right adrenal gland appeared normal. The liver was enlarged and hyperechoic.
Ask Yourself:1. What was the most likely diagnosis, and what additional testing should be performed to determine the cause?
2. What caused divergent results on the ACTH stimulation and low-dose dexamethasone suppression tests?
3. What additional diagnostics should be considered to obtain a complete clinical picture?
Diagnosis: Hyperadrenocorticism
Although the ACTH stimulation test was within the reference range, results of the low-dose dexamethasone suppression test were consistent with a diagnosis of hyperadrenocorticism (HAC). This is particularly notable considering the other supportive findings of polyuria and/or polydipsia, polyphagia, dermatologic signs, increases in ALP and ALT activities, hypercholesterolemia, thrombocytosis, and hyposthenuria.
Related Article: Low-Dose Dexamethasone Suppression Testing for Hyperadrenocorticism
Treatment OptionsThe definitive treatment for an adrenal tumor is surgical removal; however, adrenalectomy is not a straightforward procedure and has a significant mortality rate. Approximately 50% of adrenocortical tumors are benign adenomas; the other half are malignant carcinomas. Before pursuing surgery, potential metastatic disease should be investigated via thoracic radiography and abdominal ultrasonography. CT scan of the abdomen can also aid surgical planning.
Ideally, HAC is first controlled medically to minimize chance of surgical complications. Trilostane (Vetoryl, dechra-us.com) and mitotane (Lysodren) are two commonly prescribed medications. Ketoconazole (Nizoral) is another option.
Dosing schemes for PDH may not effectively treat a functional adrenal tumor. If dose escalation is required, it should be executed cautiously with close monitoring. If surgery is not pursued, long-term medical therapy with these medications is a good alternative. Favorable results have been reported with mitotane or trilostane.1-3
Before surgery was pursued, this dog was treated with trilostane at 1 mg/kg PO q12h. Benazepril was also administered at 0.25 mg/kg PO q12h to address proteinuria and hypertension. After 2 weeks, ACTH stimulation testing showed insufficient control, and clinical signs persisted. The dose of trilostane was increased by 25% to 1.25 mg/kg PO q12h.
Two weeks later, clinical signs were markedly improved, ACTH stimulation testing showed adequate control of HAC, and systolic blood pressure had decreased to 140 mm Hg. Left adrenalectomy was performed. Recovery was uneventful. Histopathologic examination of the adrenal nodule revealed a benign adrenocortical adenoma.
Follow-up testing (ie, chemistry panel, CBC, ACTH stimulation test) showed that the hyperadrenocorticism and associated signs had resolved. Benazepril was discontinued.
After 1 month, the systolic blood pressure measured by Doppler ultrasonography was 110 mm Hg and urine protein:creatinine (UP:C) ratio was 0.4.
Related Article: Update on the Use of Trilostane
Did You Answer?
1. The nodule on the left adrenal gland suggested that a functional adrenocortical tumor was the underlying cause of HAC, but additional investigation is required to rule out the more common pituitary-dependent hyperadrenocorticism (PDH).
Approximately 80%–85% of dogs with HAC have PDH; only 15%–20% have an adrenocortical tumor.4 In some dogs with PDH, adrenal hyperplasia caused by excessive ACTH stimulation can appear as a nodule. Measurement of endogenous ACTH concentration and a high-dose dexamethasone suppression test can help differentiate PDH from adrenocortical tumor.
This dog had an endogenous ACTH concentration of <10 pg/mL, consistent with a functional adrenocortical tumor as the source of HAC.
Related Article: Anesthesia for Adrenal Gland Disease
2. The divergent results on ACTH stimulation and low-dose dexamethasone suppression testing can be understood after the discovery of the functional adrenocortical tumor. The reported sensitivity of the ACTH stimulation test for detecting this HAC is 61%, indicating that false-negative results are common.5 The neoplastic cells in adrenal tumors are abnormal, and although they produce excessive cortisol, they seldom do so in response to ACTH. Therefore, it is not surprising to find a negative ACTH stimulation test with this type of HAC.
The exact sensitivity of the low-dose dexamethasone suppression test for diagnosing adrenocortical tumor has not been reported, but the sensitivity of this test for all types of HAC is 95%. If an ACTH stimulation test is conducted in the diagnostic investigation for HAC, a negative result should be interpreted with caution.
3. Dogs with HAC can have clinically significant comorbid conditions resulting from the effects of increased steroid hormone concentrations. Additional diagnostic testing is recommended.
One study found that 46% of dogs had a urinary tract infection at HAC diagnosis, so a urine culture was indicated.6 Of these 46%, fewer than 5% exhibited signs of an infection (eg, pollakiuria, stranguria), and the expected increased wbc counts and bacteria in the urine may not have been present because of the effects of cortisol and urine dilution.
A UP:C should be obtained, but only after the urine culture result is negative to rule out infection as the cause of proteinuria. Hypertension has also been associated with HAC; 86% of dogs have had increased blood pressure at diagnosis.7 To document hypertension, blood pressure should be measured on at least two occasions.
This dog had a negative urine culture and a UP:C of 2.8. Its systolic blood pressure measured by Doppler ultrasonography was 180 mm Hg.
Another test to be considered is thromboelastography (TEG). Relatively new in veterinary medicine, TEG has limited availability but is gaining acceptance as a method to evaluate hypercoagulability.
Canine HAC has been associated with thromboembolic complications (eg, pulmonary thromboembolism) that can be severe, and TEG for the detection and treatment of this problem is an active area of research. One study found that 80% of a small population of dogs with HAC had hypercoagulability based on TEG.8 However, the precise role of TEG in treatment is still evolving.
A TEG was conducted in this case, and the results were normal. Hypercoagulability was not present on TEG; if it were, treatment with anticoagulants could be considered, but the decision is currently subjective and made on a case-by-case basis.
MARY ANNA LABATO, DVM, DACVIM (Small Animal), is clinical professor and section head in small animal medicine at Tufts University, where she also earned her DVM. She has a special interest in renal disease and interventional therapies. Dr. Labato has numerous journal publications.
BENJAMIN NOLAN, DVM, PhD, DACVIM (Small Animal), is staff internist at Aspen Meadow Veterinary Specialists in Boulder, Colorado. He has a special interest in renal and endocrine disease. He completed a one-year internship at Alameda East Veterinary Hospital in Denver and his residency at Tufts University, where he also earned his DVM.
POLYURIA & POLYDIPSIA IN A DOG • Benjamin Nolan & Mary Anna Labato
References
1. Trilostane treatment of a dog with functional adrenocortical neoplasia. Eastwood JM, Elwood CM, Hurley KJ. J Small Anim Pract 44:126-131, 2003.2. Trilostane therapy for hyperadrenocorticism in three dogs with adrenocortical metastasis. Benchekroun G, de Fornel-Thibaud P, Lafarge S, et al. Vet Rec 163:190-192, 2008.3. Mitotane treatment of 32 dogs with cortisolsecreting adrenocortical neoplasms. Kintzer PP, Peterson ME. JAVMA 205:54-61, 1994.4. Canine hyperadrenocorticism (Cushing’s syndrome). In Feldman EC, Nelson RW (eds): Canine and Feline Endocrinology and Reproduction, 3rd ed−St. Louis: Saunders Elsevier, 2004, pp 252-357.5. Diagnosis of canine hyperadrenocorticism. Behrend EN, Kemppainen RJ. Vet Clin North Am Small Anim Pract 31:985-1003, 2001.6. Systemic arterial blood pressure and urine protein/creatinine ratio in dogs with hyperadrenocorticism. Ortega TM, Feldman EC, Nelson RW, et al. JAVMA 209:1724-1729, 1996.7. Retrospective evaluation of urinary tract infection in 42 dogs with hyperadrenocorticism or diabetes mellitus or both. Forrester SD, Troy GC, Dalton MN, et al. JVIM 13:557-560, 1999.8. Effect of canine hyperadrenocorticism on coagulation parameters. Rose L, Dunn ME, Bédard C. JVIM 27:207-211, 2013.
Suggested Reading
Adrenalectomy in dogs with adrenal gland tumors: 52 cases (2002-2008). Massari F, Nicoli S, Romanelli G, et al. JAVMA 239:216-221, 2011.
Trilostane in dogs. Ramsey IK. Vet Clin North Am Small Anim Pract 40:269-283, 2010.