Performing Renal Biopsy
George E. Lees, DVM, MS, Diplomate ACVIM, Texas A&M University
Anne Bahr, DVM, MS, Diplomate ACVR, Texas A&M University
Mary H. Sanders, RVT, Texas A&M University
Performing a renal biopsy requires selecting a suitable method of approaching or locating the target kidney and choosing a device or method to retrieve the tissue. Ultrasonography-guided needle biopsy techniques are commonly used and are generally satisfactory when the expected changes are likely to be diffusely distributed in the cortex (as for acute kidney injury and most glomerular disorders).
Biopsy Needles
A variety of automated biopsy devices that procure satisfactory specimens when used properly are available commercially. The diameter and depth of penetration (ie, length of throw) of the needle should be appropriate for the size of the target, which can be assessed directly with ultrasonographic guidance.
When the cortex is thin (because the animal is small or because of the effects of disease), use of a short-throw needle (eg, 11-mm throw, 7-mm specimen notch) is recommended to help keep the biopsy tracts entirely within the cortex, even if taking a few more samples is necessary to obtain sufficient tissue.
If the samples are intact (not fragmented) and handled carefully, the cores provided by 18-gauge needle biopsy devices generally are satisfactory for most purposes. Nonetheless, all other things being equal, larger-diameter needles (eg, 16-gauge) yield more informative samples and are preferable when they can be used safely.

Use a dissecting microscope with 20× to 40× magnification to assess the tissue composition of each biopsy core retrieved.
Patient Positioning
There are several different combinations of patient positions, scanning angles, needle directions, and aspects of the kidney to be biopsied that can be used successfully depending on operator preferences. The approach should minimize the possibility of inadvertent damage to the major renal vessels in the hilus because this can lead to a catastrophic outcome, such as fatal hemorrhage.
Some prefer to perform a biopsy of the right kidney because it typically is less mobile (ie, held in place against the liver) than the left kidney; however, others prefer the left kidney because of its more caudal and superficial location.
Technique
A biopsy guide attached to the scanning probe to direct the needle biopsy device is the safest method, but some people prefer to use a “freehand” technique in which the probe and biopsy device are not connected to one another and can be manipulated independently until the operator is certain that the needle is positioned optimally. This latter method requires excellent hand–eye coordination and extensive operator experience. Operators should have extensive practice in aspiration and biopsy of other organs and masses before attempting kidney biopsy because there is little margin for error.
Author Insight
Ultrasonography-guided needle biopsy of the kidney should be performed under general anesthesia to allow sufficient control over patient discomfort and motion, including respiratory motion, during the procedure.
Step-by-Step: Renal Biopsy
What You Will Need
Shown here is the top of a mobile cart used at Texas A&M University to help process renal biopsy specimens. The cart is readily taken to the site in the hospital (eg, surgery or ultrasound suite) where specimen collection is planned.
The dissecting microscope (or some other suitable means of magnification and good illumination) is used to assess specimen content. The forceps have no teeth and are suitable for delicate manipulation of the samples. The syringe containing sterile saline and fitted with a 25-gauge needle irrigates the specimens after collection. The ice bucket (or a nearby refrigerator) helps keep the fixative for electron microscopy chilled. The rest of the items displayed are provided in renal biopsy kits that are available from centers that perform comprehensive pathologic evaluations.

Step 1
The cortex of the kidney is the proper target for all renal biopsy procedures for 2 important reasons, the first of which is safety. Biopsy needle tracts that cross the corticomedullary junction are associated with risk for damaging the large vessels (eg, arcuate arteries) that are located there, possibly causing both excess hemorrhage and greater damage to the renal parenchyma as a result of ischemia or infarction of the region served by the damaged vessel.
Second, the renal cortex is the primary tissue of interest for almost all indications for kidney biopsy. Indeed, all glomeruli are in the cortex, and a renal biopsy for evaluation of glomerular disease is inadequate if it does not contain a large enough sample of cortical tissue.

Step 2
The lateral cortex (if imaging is done in a ventrodorsal position) or the dorsal cortex (if imaging is done in a lateral position) is often the best area from which to obtain samples based on the availability of ultrasonographic windows and ability to position the biopsy device appropriately. These areas also are distant from the hilus, which minimizes the risk for damaging the major vessels located there.
Although a scan plane that includes both the cortex and medulla often makes the kidney easy to recognize, a plane that includes only the cortex (in which the biopsy tract will be confined) is recommended for renal biopsy. The kidney typically is visualized in a sagittal or dorsal plane, after which the operator should fan the scan plane so that only the cortex remains in the plane in which the biopsy needle will be placed.
Author Insight
Specimens should be kept moist (never placed on dry sponges) with physiologic saline solution and manipulated very gently (without grasping them with forceps) as they are collected, assessed, processed, and placed in fixative or preservative.
Step 3
When the scan plane and direction of needle placement have been identified, a small stab incision is made through the skin at the entry point to minimize dulling of the biopsy needle before it is advanced through the body wall to the kidney.
In addition, and before activation of the biopsy device, it often is necessary to advance the biopsy needle tip into the capsule of the kidney (especially when biopsy of the left kidney is being performed) to minimize movement of the kidney away from the biopsy instrument when it is activated.
Step 4
In general, it is best to collect at least 2 cortical cores if each is > 10 mm long. When the cores are shorter than 10 mm each, 3 cores are usually required. Needle biopsy cores do not need to be cut into smaller pieces except as needed to subdivide them for separate evaluations.

Step 5
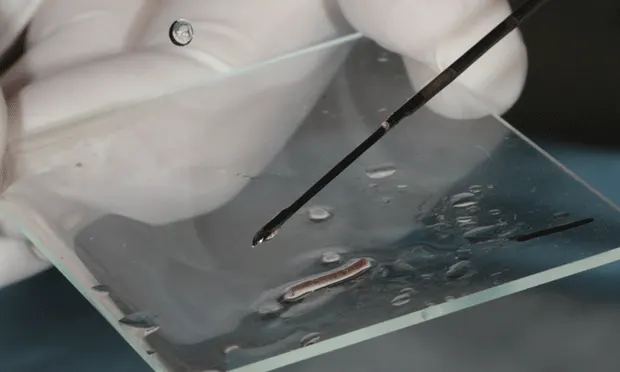
The renal tissue core can be retrieved from the needle biopsy device by using a gentle flow of sterilized normal saline solution through a 25-gauge needle to wash the specimen onto the glass slide. Using this technique prevents the sterile biopsy needle from touching the glass slide. After the specimen is retrieved, vigorous flow of saline can be used to dislodge tissue tags that remain in the specimen notch before the biopsy needle is reused to obtain an additional core.
Author Insight
Assess the tissue content of the cores to verify that satisfactory samples have been obtained before ending the biopsy procedure.
Step 6
Visual assessment of the composition of needle biopsy tissue cores to verify that they contain glomeruli (ie, that the samples to be submitted for evaluation are cortical tissue) is an important, often overlooked step. This is best accomplished with a low level of magnification (10–40×), such as can be achieved with good illumination and a dissecting microscope, ocular loupe, or hand-held lens.
With such magnification, several aspects of the appearance of core biopsy specimens help differentiate the cortex from the medulla. One is that glomeruli often can be seen in cortical tissue as small spherical structures or merely as spherical disruptions in the surrounding pattern of tubules. However, even when individual glomeruli are not recognized, the cortex can usually be distinguished from the medulla on the basis of the general architecture of the tissue: tubules in cortical tissue are convoluted (they appear jumbled) (Figure A), whereas those in the medulla are straight and parallel with one another (Figure B).


Step 7
Samples needed for electron microscopic examination or immunostaining must be appropriately processed and placed into the proper fixatives and preservatives when tissue specimens are first obtained.
Figure A shows the recommended ways to subdivide renal biopsy cores for light microscopic (LM), transmission electron microscopic (TEM), and immunofluorescence (IF) evaluations under the following conditions: (1) the cores are not examined with sufficient magnification to allow direct assessment of their composition, (2) the cores are verified to be composed of cortex and 2 long (> 10 mm) cores are available, and (3) the cores are verified to be composed of cortex and 3 short (< 10 mm) or fragmented cores are available.
Figures B through D show division of a needle biopsy core into 2 portions for separate evaluations (one for TEM evaluation and the other for IF microscopic evaluation) after examination with sufficient magnification to verify that the core is composed entirely of cortical tissue.

FIGURE A
Step 8
Centers that perform renal biopsy evaluations provide kits containing the materials and instructions needed to obtain, process, and submit satisfactory renal biopsy specimens to their laboratories. For further information on renal biopsy kits and the evaluation process, see Evaluation of Renal Biopsy Samples (October 2009).

Wedge Biopsies
Needle biopsy devices also can be used with other aiming methods, including manual palpation (such as in cats), laparoscopy, and a keyhole or fully open celiotomy. However, when surgeons use biopsy needles to obtain specimens of the kidney during a celiotomy, they often direct the devices too deeply. This problem is avoided if a wedge biopsy sample is obtained. In addition, wedge biopsies are better for evaluating renal changes that are not uniformly distributed in the cortex, as often is the case in animals with juvenile-onset renal diseases.
