Oxygen Therapy in Dogs & Cats
Lori S. Waddell, DVM, DACVECC, University of Pennsylvania
How can I provide oxygen supplementation for small animal patients?
Oxygen therapy is indicated for any patient presented in respiratory distress. Even a patient with mild-to-moderate respiratory distress should receive supplemental oxygen. Patients in severe distress require oxygen supplementation immediately, before attempts to identify the cause. If quantitative measurements are available, oxygen supplementation should be provided to any patient with oxygen saturation (SaO2) or pulse oximetry reading (SpO2) of <93% or with an arterial partial pressure of oxygen (PaO2) of <80 mm Hg.1 Minimal risk is associated with short-term oxygen supplementation, which rapidly benefits most hypoxic patients. Oxygen supplementation can be provided while a quick patient assessment is performed. This includes evaluation for upper airway obstruction that may require immediate treatment with endotracheal intubation or tracheostomy. Oxygen supplementation should also be provided for patients with respiratory distress during catheter placement, thoracocentesis, radiography, or sedation for any other procedures.
Oxygen can be supplied from various sources (eg, centralized in-house oxygen, portable oxygen tanks, anesthetic machines) and in many different ways, depending on the severity of respiratory distress, the need to handle the patient while providing oxygen, the duration supplementation is needed, the available equipment, and the clinical experience and skills of the clinician (see Table).2
Table: Oxygen Supplementation Methods
Flow-By Oxygen
Flow-by oxygen is fairly nonstressful for the patient and is achieved by holding the oxygen supply tubing near the patient’s nostrils or mouth (Figure 1) and using high flow rates (2-15 L/min). This may mildly increase the patient’s percentage of inspired oxygen concentration from 21%-25% to 40%, with flow rates of 2 to 3 L/min if the tubing is held adjacent to or within 2 cm of the patient’s nostrils.3 However, this method requires the tubing to be held in place and therefore is best suited for short time periods (eg, for patient assessment, during a procedure). Unattended flow-by oxygen should not be used, as subtle patient movements can render it ineffective.
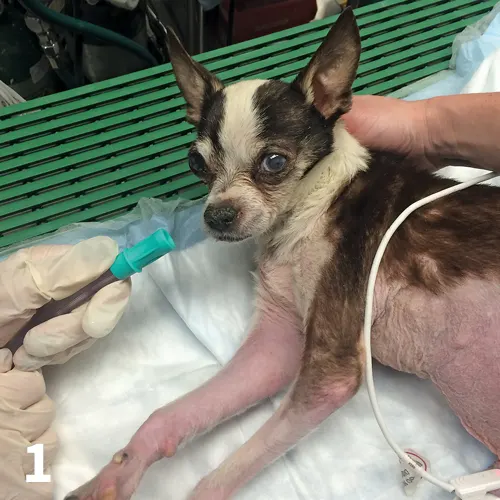
FIGURE 1 A dog receives flow-by oxygen to allow for examination and monitoring.
Mask Oxygen
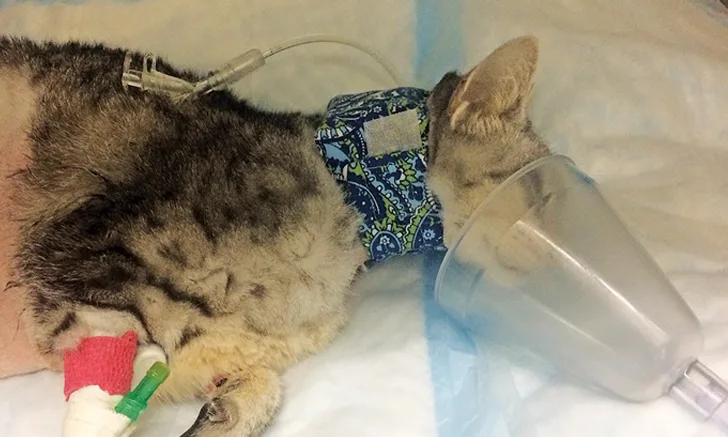
Mask oxygen can be used on any patient that will tolerate the mask and should be avoided in distressed patients, as persistent attempts to place the mask over the muzzle can increase stress. This technique requires minimal equipment and can be quite effective in recumbent patients (eg, those recovering from anesthesia or sedation; Figure 2); it also allows for delivery of oxygen supplementation while the patient is being handled for examination and treatment. With a tight-fitting mask at high oxygen flow rates (8-12 L/min), oxygen concentrations of 50% to 60% can be achieved.3 Care must be taken to ensure the mask is not tight enough to cause rebreathing of carbon dioxide and consequent hypercarbia.
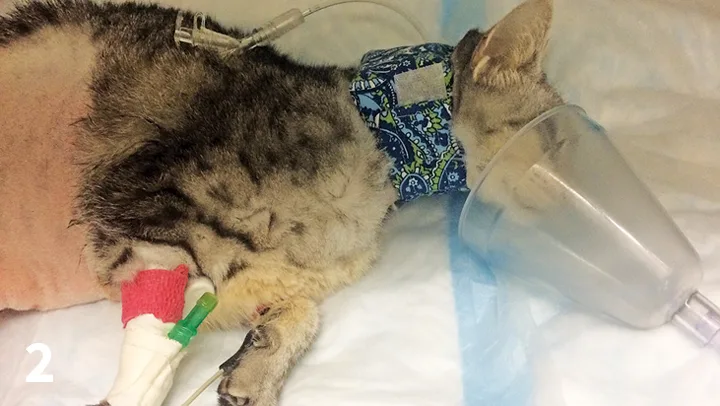
FIGURE 2 A cat receives supplemental oxygen via mask during recovery from anesthesia.
Oxygen Hood
An oxygen hood can be created by using oxygen tubing to supply oxygen to an Elizabethan collar covered with plastic wrap, leaving 2.5 to 5 cm open at the top of the Elizabethan collar to allow for carbon dioxide and water vapor elimination (Figure 3). This allows the animal to be visible and can minimize stress. Oxygen concentrations of ≈30% to 40% with flow rates of 0.5 to 1 L/min are possible after the hood has been initially filled with oxygen at a higher rate (1-2 L/min).4 This technique is not tolerated by all patients but can be effective for oxygen delivery in some, especially if oxygen cages are not available. The humidity and temperature in the enclosed space of the Elizabethan collar may increase rapidly. Therefore, frequent monitoring of the patient is required and the duration for which this technique can be used may be limited. Additionally, carbon dioxide can accumulate if the hood is not vented properly.
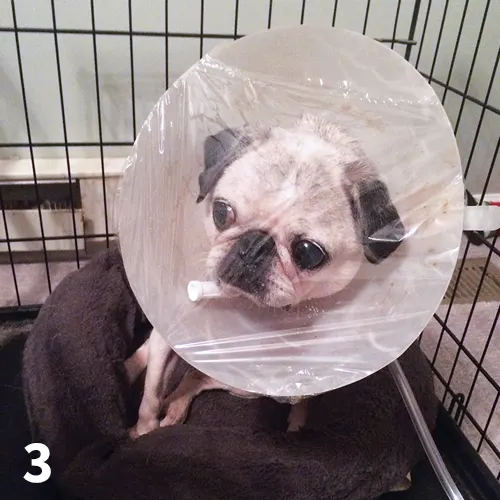
FIGURE 3 A pug receives oxygen supplementation via oxygen hood.
Oxygen Cage
Oxygen cages are among the easiest methods of oxygen administration; however, they isolate the patient, which sometimes makes it difficult to examine, monitor, and treat these dynamic cases (Figure 4). For some patients (eg, stressed cats), isolation from a strange environment and humans may be beneficial. Initial crisis management of animals in respiratory distress may require high oxygen concentrations (up to 90%) until the patient has been stabilized. It should be possible to reach 100% oxygen. However, in the author’s experience, limitations of oxygen cages include: the inability of some oxygen cages to raise the concentration above ≈50%, which may be too low for severely dyspneic animals; opening the cage door drops the oxygen concentration to that of room air almost immediately; oxygen cages may be associated with inappropriate patient warming and secondary hyperthermia (ice packs in the cage can help control temperature); and large dogs may not fit in standard oxygen cages. Despite these limitations, oxygen cages are often useful for emergency practices. Pediatric incubators, into which oxygen is piped, provide a suitable alternative for cats, small dogs, and neonates; in the author’s experience, they attain oxygen concentrations of 80% to 90%. These allow for good observation of the patient and, although expensive when purchased new, are often available secondhand from human hospitals. Oxygen sensors can measure the oxygen concentration in incubators or other makeshift oxygen cages.

FIGURE 4 A dog in severe respiratory distress in an oxygen cage.
Nasal Oxygen
For more prolonged oxygen supplementation, nasal oxygen can be provided with nasal oxygen prongs manufactured for human patients (Figure 5) or by placing a catheter in 1 or both nostrils and suturing or gluing it in place (Figure 6). Nasal prongs penetrate ≈1 cm into both nostrils and typically work well in large-breed dogs that are relatively immobile. Nasal prongs likely supply oxygen levels similar to those supplied by the flow-by technique.
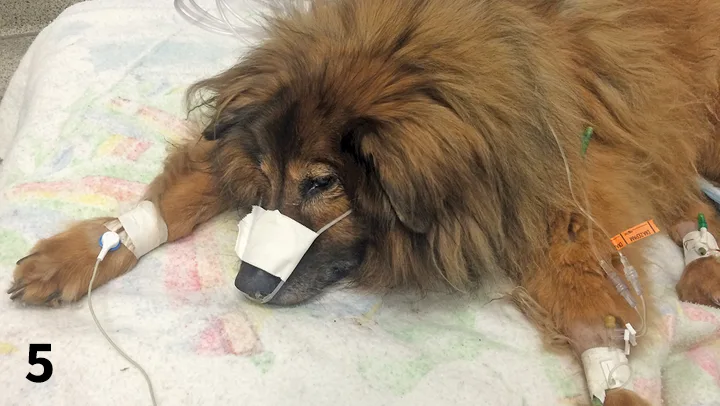
FIGURE 5 A dog with aspiration pneumonia receives oxygen supplementation via nasal prongs.

FIGURE 6 A Labrador retriever with a nasal catheter placed for oxygen supplementation
Nasal catheters can be used in dogs or cats of almost any size. Any type of catheter can be used for this purpose, but 5-8 French red rubber urinary catheters or soft feeding tubes are more common. The catheter should be premeasured from the nostril to the medial canthus of the eye. This will place the tip of the catheter into the nasopharynx. A few drops of a local anesthetic (eg, topical 2% lidocaine) should be placed in the nostril. The tip of the tube should be lubricated with lubricating jelly or lidocaine gel. The tube should be placed medially to the premeasured length then sutured at the alar fold of the nostril and again on the side of the patient’s face or over the top of the head.5 An Elizabethan collar is often needed to prevent the patient from rubbing out the catheter.
Brachycephalic breeds are poor candidates for nasal oxygen supplementation because catheter prongs cannot be adjusted to fit comfortably on their faces; nasal catheters may not fit because of stenotic nares nor stay in place because of these patients’ short noses; and increased vagal tone may be aggravated by the nasal catheter (author experience). Animals that are mouth breathing are also poor candidates, as increased air mixing in the pharynx leads to a reduced effective oxygen concentration. Generally, nasal oxygen is thought to give an oxygen concentration of ≈40%, depending on the flow rate of oxygen, the size of the animal, and its minute ventilation.6 The oxygen concentration can be increased by placing a second nasal catheter in the other nostril. With bilateral nasal catheters, oxygen concentration of up to 60% can be attained with a flow rate of 100 mL/kg/min and up to 80% at a flow rate of 200 mL/kg/min.6
Alternatively, a long IV catheter can be placed percutaneously into the trachea following local anesthesia. Humidified oxygen supplied at rates of 50 to 150 mL/kg/min can be used and may obtain oxygen concentrations of 40% to 60%.7 A more detailed description of this technique can be found in Advanced Monitoring and Procedures for Small Animal Emergency and Critical Care.5
Other Considerations
If the patient is in severe respiratory distress or has an upper airway obstruction, it may be necessary to secure an airway by intubating with an endotracheal tube (Figure 7). This will allow more cranial obstructions (oropharyngeal, laryngeal, majority of the trachea) to be bypassed if the tube can be placed through the obstructing area. It will also allow for supplementation with 100% oxygen if required and mechanical ventilation for patients with severe pulmonary disease or an inability to ventilate because of neuromuscular causes. These patients require constant supervision and monitoring while intubated.
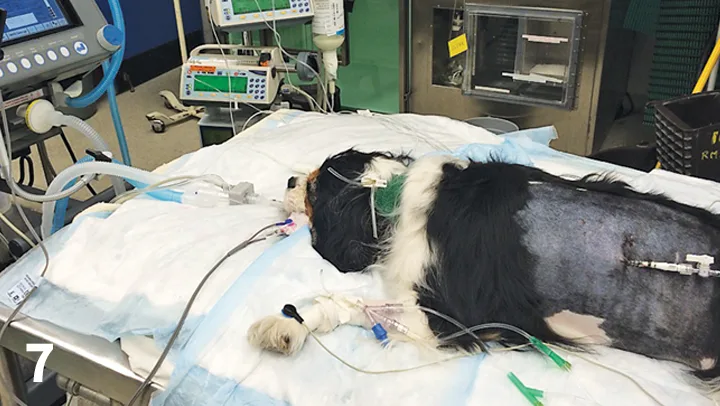
FIGURE 7 After being hit by a car, a Cavalier King Charles spaniel is intubated for mechanical ventilation because of severe pulmonary parenchymal disease.
If being provided for more than a few hours, oxygen should be humidified (ie, saturated with water vapor) to prevent desiccation of the airways, especially if the turbinates are bypassed with nasal or tracheal oxygen catheters. Specially designed units that heat and humidify inspired air are available for placement in anesthetic and ventilator circuits, but nasal or cage oxygen humidification can be accomplished by bubbling the oxygen through a chamber of distilled water.
Patients receiving oxygen therapy should be monitored closely using physical examination parameters such as respiratory rate and effort, mucous membrane color, and heart rate, as hypoxemia can cause tachycardia. Pulse oximetry or arterial blood gas analysis can be used to confirm the patient is oxygenating at an acceptable level with oxygen supplementation. Pulse oximetry is the easiest, least invasive method. The lowest oxygen concentration that maintains the patient at SpO2 >93% or PaO2 >80 mm Hg should be used. As the underlying condition improves, oxygen supplementation should be slowly decreased while ensuring adequate oxygenation. If adequate oxygenation cannot be attained with supplemental oxygen at an acceptable or attainable level, intubation and positive pressure ventilation with positive end-expiratory pressure should be considered. Long-term therapy with high concentrations of oxygen (100% O2 for >24 hours or 60% O2 for >48 hours) is associated with lung damage (ie, oxygen toxicity).8 Inflammatory injury is caused by toxic metabolites of oxygen, including oxygen free radicals and superoxide molecules. Clinically, oxygen toxicity is difficult to diagnose, but changes in the lungs are similar to those seen in acute respiratory distress syndrome.8 The oxygen concentration used to maintain critical patients should always be minimized to the lowest the patient can tolerate.
Editor's note: This article was originally published July 2016 as "Oxygen Therapy"