
Max, a 15-year-old, 18.2-lb (8.3-kg) neutered male Maine coon, was referred for evaluation of chronic pain attributed to osteoarthritis (OA), which had been diagnosed by the referring clinician 7 months prior. The owner reported Max had trouble ambulating, experienced pelvic limb collapse, and would bite when touched on the caudal end.
Pain persisted despite weight loss and initial management that included administration of compounded gabapentin (12 mg/kg PO every 12 hours for pain management), methimazole (2.5 mg/cat PO every 12 hours to treat previously diagnosed hyperthyroidism), glucosamine/chondroitin (1 capsule [per package label] PO every 24 hours for joint health), polysulfated glycosaminoglycan (2 mg/kg SC every 1-2 weeks as a disease modifying drug for OA), a homeopathic remedy for pain relief, and a nutritional supplement for managing inflammation; cold laser therapy (elbows, hips, and knees) was also used.
Physical Examination
Physical examination was performed with Max under sedation due to his fractious temperament. BCS was 9/9. There was significant disuse muscle atrophy of the pelvic limbs; palpable crepitus in both stifles and elbows; and decreased extension, decreased range of motion, and discomfort on extension of the right hip. The left hip had appropriate range of motion and no crepitus. Crepitus without instability was noted at the level of the stifles. The remainder of the examination was unremarkable.
Diagnosis
Radiographic Findings
Radiographs taken by the referring clinician showed a shallow right acetabulum with decreased coverage of the femoral head and secondary changes, including a sclerotic acetabular rim, smoothly marginated periosteal proliferation along the cranial and caudal pillars of the acetabulum, flattening of the right femoral head, thickening of the femoral neck, and mild craniodorsal subluxation. The left coxofemoral joint was congruent but had mild changes with osteophytes along the cranial acetabular margin. There was a mild amount of spondylosis deformans at L6-L7 and L7-S1. Radiographs of the stifles showed subchondral bone sclerosis of both medial femoral condyles and medial tibial condyles. Osteophytes and enthesophytes were present along the distal femurs and proximal tibiae. A large amount of amorphous periarticular new bone was located within and adjacent to the craniomedial aspects of the stifle joints and just proximal to the tibial eminences. Radiographic diagnoses at that time were moderate, right-sided, coxofemoral degenerative joint disease with subluxation of the femoral head; mild, left-sided, coxofemoral degenerative joint disease with no evidence of subluxation; and severe, bilateral stifle degenerative joint disease.At the referral clinic, 8.5 months after initial presentation, Max was sedated, and repeat ventrodorsal and orthogonal lateral pelvic radiographs were obtained to evaluate progression or change (Figure).
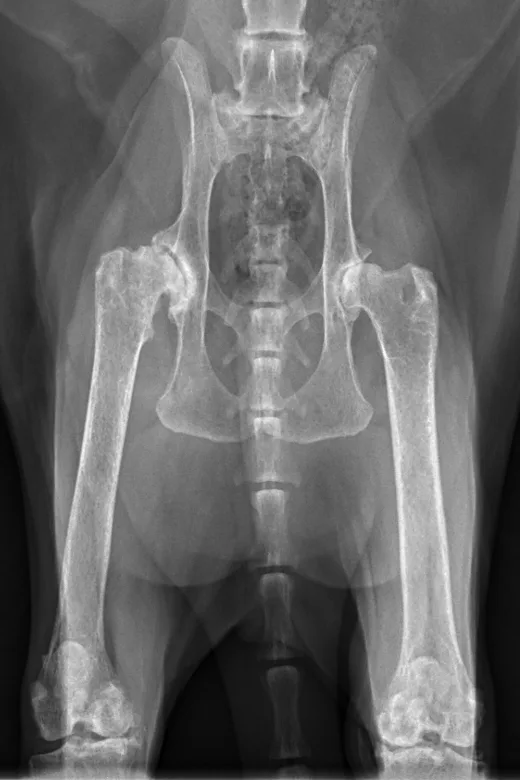
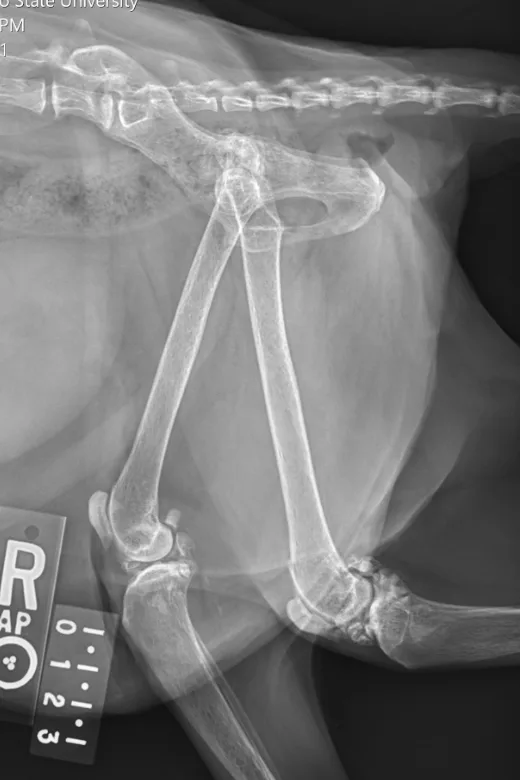
Ventrodorsal and lateral pelvic radiographs showing severe osteophyte formation along the right femoral head and neck that caused effacement of the trochanteric fossa. The acetabulum is shallow due to remodeling and osteophyte formation. Mild osteophyte formation remains along the left femoral head and neck, as well as moderate osteophyte formation along the acetabulum. Caudal lumbar spondylosis deformans is unchanged from initial radiographic findings. Rounded, angular mineral bodies are present bilaterally in the stifle joints with moderate concurrent periarticular osteophyte formation. Short, linear mineral bodies can be seen in the soft tissues of the right caudal crus.
Although findings were unchanged from previous radiographs, OA in the right hip appeared mildly progressed.
Differential Diagnoses
Differential diagnoses for decreased range of motion with crepitus in the hip include primary degenerative joint disease, degenerative joint disease secondary to hip dysplasia, fracture (acute or chronic), Legg-Calve-Perthes disease, hip luxation, infective/inflammatory arthritis, and neoplasia.
Bilateral hip dysplasia with secondary OA and bilateral stifle OA were diagnosed based on patient history, physical examination findings, and survey radiographs.
Diagnosis: Hip Dysplasia With Secondary & Stifle Osteoarthritis
Treatment & Management
Max was continued on the medication regimen outlined previously. Rehabilitation was initiated, including acupuncture, low-level laser therapy (12 J/cm2 applied to the lumbar spine, hips, and stifles), pulsed electromagnetic field therapy, soft-tissue techniques (eg, massage, stretching, passive range of motion, joint mobilization), and exercise on an underwater treadmill. Massage and passive range of motion were performed without sedation.
The first rehabalitation session went well, and weekly sessions were advised. The importance of activity to maintain joint health and encourage weight loss were explained to Max’s owner, and recommendations were made on how to encourage activity at home, including spending more time outside.
Underwater treadmill time and exercises were increased as tolerated; the amount and duration of therapy were dictated by Max’s comfort and willingness to comply. Max had improved hip comfort and range of motion by the third rehabilitation session. He was increasingly comfortable and compliant at each session, and his temperament improved as pain was better controlled by the previously outlined multimodal approach.
Weekly rehabilitation therapy sessions continued, as well as meloxicam (0.04 mg/kg PO every 72 hours; initiated 2 years after rehabilitation therapy was started, extra-label with owner consent), gabapentin (50 mg/cat PO every 12 hours when at home for pain management; 100 mg PO at the time of drop off when coming to the clinic, at least 2 hours prior to the beginning of treatment, as gabapentin is shown to cause the greatest reduction of stress 2 hours postadministration), polysulfated glycosaminoglycan (4.45 mg/kg SC every 7 days), glucosamine/chondroitin (1 capsule [per package label] PO every 24 hours), methimazole (5 mg/cat PO every 12 hours; dose based on thyroid level), acupuncture, low-level laser therapy (12 J/cm2 applied to the lumbar region, hips, and stifles), pulsed electromagnetic field (60 minutes during laser therapy and acupuncture), soft-tissue techniques (eg, massage, stretching, passive range of motion, joint mobilization), underwater treadmill use (intermittent and patient temperament dependent), and therapeutic exercises (variable).1,2
TREATMENT AT A GLANCE
Gabapentin and meloxicam can be used to control pain.
Oral glucosamine/chondroitin and polysulfated glycosaminoglycan injections can be administered as supplemental therapy.
Increased activity at home can aid weight loss.
Rehabilitation therapy that includes acupuncture, photobiomodulation therapy (eg, class IV laser), pulsed electromagnetic field therapy, soft-tissue techniques, underwater treadmill use, and therapeutic exercises can be beneficial.
Prognosis & Outcome
Continued therapy and medication helped with long-term control of Max’s pain, enabling comfort and continued mobility gain.
After 2 years, Max’s weight was 8.4 lb (3.8 kg; 54% decrease from initial presentation); this significant weight loss was likely a key component of improvement. Max ambulated freely in the home, could go up and down stairs, went outside, and played with his feline housemate. He also tolerated being touched on the caudal end and had an improved temperament.
Discussion
Cats and dogs with OA can have different presentations. Dogs often develop OA because of an underlying condition (eg, joint dysplasia, cruciate disease, luxating patellae) that can lead to joint pathology and pain.3 Cats are often presented with primary OA (ie, no underlying disease causing pathology); in one study, a potential underlying cause of OA could only be found in 11% of cats.4
Cats often do not show abnormalities related to OA on physical examination, making diagnosis challenging. Diagnosis relies heavily on signs reported by the pet owner5; it is thus important to obtain a thorough patient history and be aware of changes in the patient. Asking specific questions about comfort, jumping, grooming, and activity level at home can help assess for possible signs of OA. Awareness of the unique presentation of OA in cats can help with early diagnosis and treatment.
Radiographs can help confirm clinical signs that may be attributed to joint disease, monitor changes, and determine the degree of joint disease6; however, survey radiographs may not definitively determine the type of joint disease or inciting cause. Thorough patient history and physical examination are key to diagnosis. Arthrocentesis with cytologic evaluation can help differentiate sepsis, degenerative change, immune-mediated disease, and neoplasia if change is seen on radiographs but the underlying cause is undetermined.
OA management typically involves weight loss, activity modification with consistent exercise, and pain control. OA cannot be cured or reversed. The goal of treatment should be to manage clinical signs, including adequate pain management, and slow progression.7 Pain should be managed first, so patients can become more active, resulting in weight loss and increased mobility.7
Although NSAIDs are the mainstay treatment in dogs with OA, long-term use is limited in cats in the United States, making pain management in cats with chronic disease challenging. Many older cats with OA have concurrent renal disease, precluding use of an NSAID.
Alternative medications include gabapentin, tramadol, and amantadine. These drugs in conjunction with disease-modifying agents (eg, polysulfated glycosaminoglycan) and supplements (eg, glucosamine/chondroitin, omega-3 fatty acids) should be considered in cats with OA. Alternative methods of treatment, including rehabilitation, can also play an important role in managing chronic OA.
There are no level 1 evidence studies assessing most oral medications or laser therapy for treatment of OA pain in cats. In addition, there are few FDA-approved long-term medications available to treat OA in cats, making management significantly more challenging than in dogs.
Frunevetmab, a monoclonal antibody that targets nerve growth factor, has been FDA-approved for use in cats in the United States. Preliminary studies for this new class of pain relievers show possible treatment for feline OA pain.8,9 Clinical trials showed minimal effects on the liver, kidneys, and GI system; dermatologic adverse effects were most common.8,9 Frunevetmab is a monthly injectable medication that may be more easily administered to cats compared with daily oral medications. Further study and evaluation are needed to determine efficacy.
Surgical intervention, including total hip replacement and femoral head and neck ostectomy, is often considered in patients with hip OA, but surgery was not appropriate for this patient because of degenerative stifle changes and severe obesity.
After pain is controlled, activity can be modified to include more low-impact activities, as they are better tolerated by patients with OA. Increasing activity as tolerated should be a goal in sedentary patients.
TAKE-HOME MESSAGES
OA is common in overweight, older cats.
Feline OA is usually a primary disease process, rather than secondary to disease (eg, hip dysplasia), and routine screening is recommended.
OA cannot be cured. Treatment should focus on managing clinical signs and slowing progression.
Main principles of OA management are pain control, consistent exercise, and weight loss.
Controlling pain can help increase activity and improve weight control and should be the first step in treatment.
Listen to the Podcast
Get the author's personal insights on Clinician's Brief: The Podcast.