NSAID Therapy in Dogs
Christopher J. Jones, DVM, DACVIM, Gulf Coast Veterinary Internists, South Houston, Texas
Introduction
Nonsteroidal antiinflammatory drugs (NSAIDs) are frequently used in veterinary medicine for their analgesic, antiinflammatory, antipyretic, and antithrombotic effects. They inhibit the enzyme cyclooxygenase (COX) in the arachidonic acid pathway, resulting in inhibition of prostaglandin production. Arachidonic acid (AA), released from cell membranes following tissue damage, is metabolized by either cyclooxygenase into various prostaglandins or by 5-lipoxygenase (5-LOX) in the leukotriene pathway (Figure 1). Prostaglandins are important components of the inflammatory cascade, contributing to the vasodilation, pain, and fever associated with inflammation. They are also key mediators in a number of physiologic functions. Inhibition of their production can thus result in toxicity. Leukotrienes are mediators within the inflammatory cascade and may increase with NSAID therapy due to shunting of AA into the leukotriene pathway.1
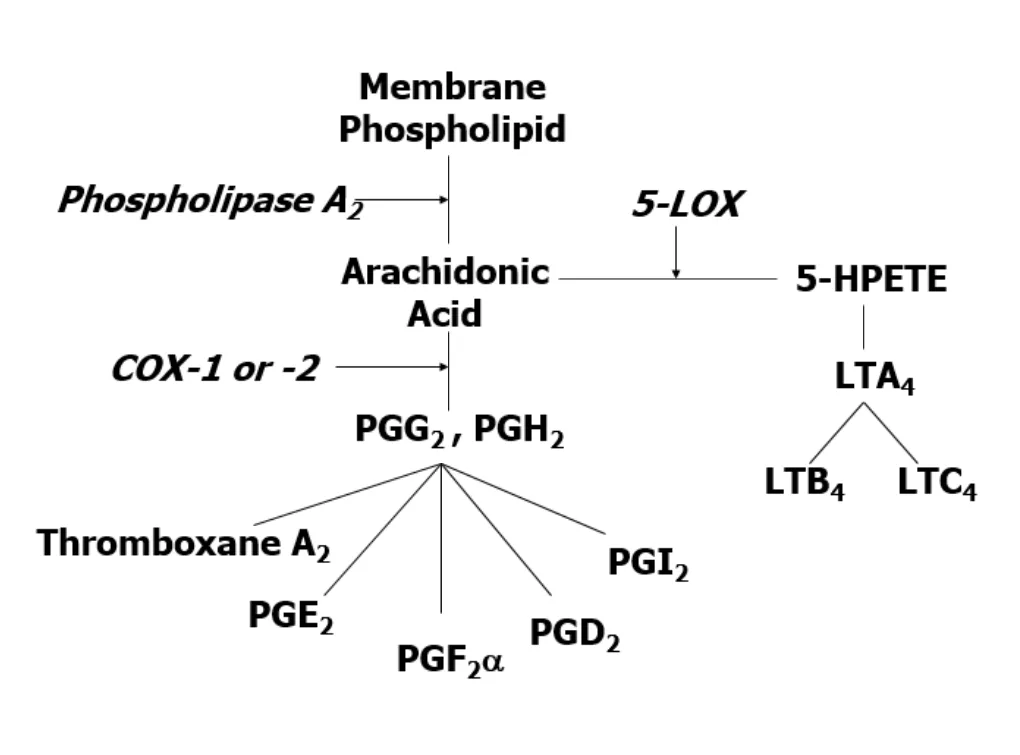
Schematic of arachidonic acid pathway (COX = cyclooxygenase; LOX = lipoxygenase; LT = leukotriene; HPETE = hydroperoxyeicosatetraenoic acid; PG = prostaglandin)
Cyclooxygenase primarily exists in two forms, COX-1 and COX-2. COX-1 has traditionally been considered the constitutive enzyme responsible for most of the basal physiologic functions of prostaglandins such as gastric cytoprotection, platelet function, and renal function. COX-2 has been considered the inducible form of the enzyme, primarily associated with inflammation. Consequently, the antiinflammatory effects of NSAIDs were originally attributed to inhibition of COX-2 while the toxic effects were attributed to inhibition of COX-1. This led to the development of more selective COX-2 inhibitors.
However, more recent data shows that this hypothesis is an oversimplification. COX-1 has been shown to play an important role in the inflammatory process.2 Conversely, COX-2 has now been isolated in a number of tissues and found to be involved in important physiologic roles including gastric healing and protection, renin release and tubular function in the kidney, vasodilation, and roles within the central nervous and reproductive systems.3 Therefore, inhibition of COX-2 may also result in toxicity, including gastrointestinal side effects (Figures 2 and 3) and renal failure.
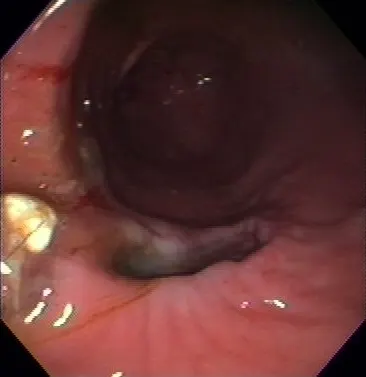
FIGURE 2
Gastric ulcer following administration of a COX-2 selective inhibitor in a dog
A third isoform, COX-3, has recently been isolated in the cerebral cortex of dogs and is a variant of COX-1.4 This isoform appears to be sensitive to the effects of acetaminophen and dipyrone.
Most currently available NSAIDs nonselectively inhibit COX-1 and COX-2, selectively inhibit COX-2, or inhibit both COX-1 and COX-2 as well as 5-LOX. This article focuses on the complications associated with NSAID therapy in dogs.
Gastrointestinal Toxicity
The GI tract is the most common site of NSAID toxicity with both nonselective and selective COX-2 inhibitors having the potential for toxicity. Prostaglandins protect the mucosa from the effects of gastric acid and other irritants through increased mucous and bicarbonate secretion, maintenance of gastric mucosal blood flow, inhibition of gastric acid secretion, and mediating gastric repair processes. Although gastric cytoprotection has been considered primarily a function of COX-1, COX-2 appears to also play a role.
One study has shown that neither a selective COX-1 inhibitor nor a selective COX-2 inhibitor induced gastric lesions in rats but when both isoforms were inhibited, gastric lesions appeared. This finding suggests that inhibition of both COX-1 and COX-2 is required for the development of gastric injury.5 COX-2 appears to be involved in maintaining gastric mucosal blood flow by decreasing leukocyte adherence in the capillaries and preventing ischemia and may also be involved in gastric adaptive responses.6 It is also necessary to promote epithelial proliferation if gastric injury occurs and inhibition of COX-2 results in delayed gastric ulcer healing.7
Prevention
The incidence of gastrointestinal toxicity in dogs is unknown and studies to determine the most effective prevention are lacking. However, some general recommendations can be made. Patients receiving NSAIDs should be well-hydrated, normotensive, and free of other systemic diseases that may result in gastrointestinal ulceration or dehydration. They should not be receiving other medications, such as corticosteroids, that may result in gastrointestinal ulceration.
Misoprostol, a prostaglandin analog, has shown benefit in preventing gastric toxicity associated with aspirin,8 but it has also been associated with side effects such as vomiting, diarrhea, and anorexia as well as abortifacient properties in humans. Side effects in animals appear to be less common.
Cimetidine, a histamine2 (H2)-blocker, is not effective in preventing aspirin-induced gastric injury in dogs.9,10 Other H2-blockers have not shown benefit in human or veterinary medicine, although ranitidine has been associated with a reduced incidence of duodenal ulceration in people.11
Omeprazole, a proton pump inhibitor (PPI), has shown success in preventing gastrointestinal ulceration associated with aspirin in dogs, but the results were not statistically significant due to the small number of dogs in the study.10 PPIs are, however, the treatment of choice to prevent NSAID gastropathy in human medicine.1 For the most part, these therapies are more effective in the treatment of NSAID-induced gastropathy once the NSAID is discontinued than they are in prevention.

Gastric ulcer near the pylorus of a dog following administration of a nonselective NSAID (naproxen)
The prevalence in humans of NSAID gastropathy has led to the development of alternative NSAIDs. The discovery of COX-2 and proposed theory that selective COX-2 inhibition could provide the antiinflammatory effects of NSAIDs without GI toxicity has led to the development of the selective COX-2 inhibitors. Many of the newer NSAIDs now used in dogs were also designed to be COX-2 selective. In humans and animals, these drugs have been shown to have reduced GI toxicity compared with nonselective NSAIDs.12,13 Furthermore, as previously discussed, COX-2 plays an important role in gastric cytoprotection and inhibition of this enzyme can lead to GI toxicity. A recent article by Lascelles documented GI perforation in dogs treated with a selective COX-2 inhibitor, although most of these dogs were treated at increased doses, for extended periods of time, in conjunction with other ulcerogenic drugs, or in the presence of other systemic diseases that may compromise gastric mucosal blood flow.14
The newer COX-LOX inhibitors are nonselective inhibitors of COX and also inhibit 5-LOX. Leukotrienes may increase with COX inhibition due to shunting of arachidonic acid into the leukotriene pathway. The leukotrienes produced may contribute to inflammation and ischemia within the gastric mucosa and thus to GI toxicity.1 The COX-LOX inhibitors may overcome this disadvantage: COX-LOX inhibitors have shown decreased GI toxicity when compared to nonselective NSAIDs in humans.1
Treatment
Treatment of gastric toxicity associated with NSAIDs requires immediate discontinuation of the medication. If a patient is having severe ulceration and hemorrhage, intravenous fluid support or blood transfusions may be necessary to maintain normal gastric mucosal blood flow. Although no studies have yet been conducted in dogs, H2-blockers or PPIs have been shown to aid gastric mucosal healing in humans once the NSAID is discontinued. Sucralfate has also been used in human medicine with similar results to ranitidine.15 These medications are also commonly used to treat GI ulceration in dogs after NSAID therapy is discontinued.
Renal Toxicity
NSAIDs have been associated with various forms of renal toxicity, including acute renal failure, interstitial nephritis, and renal papillary necrosis. Prostaglandins do not have a significant role in renal function or hemodynamics under normal circumstances, but in states of hypovolemia or hypotension they help maintain normal blood flow and GFR through renal vasodilatation, renin secretion, and decreased tubular sodium reabsorption.
Both COX-1 and COX-2 appear to be involved in these actions.16,17 Consequently, both nonselective and COX-2 selective inhibitors may result in renal compromise in hypovolemic or hypotensive situations or if used in situations in which a concurrent disease or medication may result in decreased renal perfusion. Conditions of particular concern when using NSAIDs include chronic renal failure, hepatic cirrhosis, congestive heart failure, or any condition associated with dehydration or circulatory shock. Medications of particular concern include ACE inhibitors, diuretics, and nephrotoxic agents such as aminoglycosides.
Prostaglandins have natriuretic and diuretic functions in the kidney in addition to their effects on renal blood flow, so NSAID use may result in sodium and water retention that could be significant in diseases characterized by edema formation or hypertension. In addition, NSAIDs have been shown to antagonize the effects ACE inhibitors, diuretics, and β-blockers in the treatment of hypertension.16,18 Therefore, while NSAIDs have not been reported to cause hypertension or edema in animals, they could potentially complicate therapy for these problems. Patients undergoing therapy for hypertension should be monitored closely; alternative pain management may need to be considered if they do not respond to appropriate antihypertensive agents.
Prevention
Prevention of NSAID-induced renal failure primarily involves maintaining adequate hydration. NSAIDs are unlikely to result in renal failure if adequate hydration and blood pressure are maintained. If diseases characterized by potential hypotension or hypovolemia and decreased renal perfusion are present in a given patient, NSAIDs should not be used in its treatment. Surgical patients receiving NSAIDs for pain management should be maintained on IV fluids with blood pressure monitoring to prevent the occurrence of hypotension or hypovolemia. Trauma patients should be rehydrated prior to initiation of NSAID therapy.
Treatment
Treatment of NSAID-induced renal failure requires immediate discontinuation of the NSAID. Symptomatic and supportive treatment of acute renal failure including fluid therapy, antiemetics, and H2-blockers,19 should be provided. If oliguria is present, once dehydration has been corrected, diuretic therapy with furosemide or mannitol should be initiated and dopamine may be considered for its renal vasodilating effects. If the patient is anorexic, nutritional support using appetite stimulants, nasogastric tube feeding, or parenteral nutrition may be necessary. Most patients recover if the medication is discontinued quickly and treatment is initiated before oliguria is present.
Hepatotoxicity
All NSAIDs have the potential for hepatotoxicity but the risk among various classes of NSAIDs may not be uniform. Propionic acid and phenylacetic acid derivatives seem to be associated with a higher incidence of liver toxicity.20 The toxic mechanism is generally considered idiosyncratic, the result of biotransformation of the drug to a reactive metabolite that induces an immunologic reaction or is directly toxic to hepatocytes.21 Toxicity usually occurs within 6 to 12 weeks from initiation of therapy but is not predictable or dose-dependent. Because an idiosyncratic reaction is involved, elevations of liver enzymes do not necessarily predict susceptibility to toxicity and existing liver disease does not necessarily predispose a patient to toxicity. However, liver disease may alter metabolism of the drugs, producing a reactive metabolite that leads to toxicity.
Prevention
If liver enzymes are elevated, diagnostic tests should be performed to determine the cause. Adrenal function testing and bile acid testing should be done to rule out hyperadrenocorticism and assess liver function, respectively. NSAIDs must be avoided in patients with hyperadrenocorticism until the disease is effectively managed. If liver function is impaired, radiographs, ultrasonography, and possibly a liver biopsy may be required to determine the cause of liver disease. In patients with liver disease or elevated liver enzymes, NSAIDs should be used cautiously; these patients should be monitored closely for deterioration of liver function and NSAID therapy discontinued if any signs of progression are observed. If toxicity occurs in a previously normal patient, the drug should be discontinued and, if it is severe, supportive therapy initiated. Most patients recover with discontinuation of the NSAID.
Cardiovascular Complications
NSAID therapy may have various effects on the cardiovascular system. COX-1 is responsible for thromboxane production in platelets and inhibition of this enzyme by nonselective NSAIDs may result in an increased risk of bleeding, especially during surgical procedures. Conversely, inhibition of COX-2 results in inhibition of prostacyclin, a prostaglandin important in vasodilatation and inhibition of platelet aggregation, and constitutes a potential risk of thromboembolic events.22
Dogs and cats are susceptible to thromboembolic complications associated with diseases such as cardiomyopathy, protein-losing nephropathy or enteropathy, hyperadrenocorticism, immune-mediated hemolytic anemia, and others. Although a risk of thromboembolism with COX-2 inhibition has not been documented in animals, the author believes that COX-2 selective NSAIDs should be used cautiously in patients already predisposed to thromboembolic disease.
As previously mentioned, NSAIDs may result in sodium and water retention and have been shown to antagonize the effects of ACE inhibitors, furosemide, and β-blockers.18,23 These effects may worsen hypertension or complicate therapy for hypertension and congestive heart failure. Although many of these complications are theoretical and have not been clinically reported in veterinary medicine, NSAID therapy should be monitored closely in patients undergoing therapy for hypertension, ascites, or congestive heart failure. If such patients become refractory to therapy, NSAIDs should be discontinued.
Summary
The increasingly perceived importance of pain management has resulted in an increased use of the nonsteroidal antiinflammatory drugs in veterinary medicine. With increased use of these drugs, complication rates as well as their number and types are also likely to rise. Although the more selective COX-2 inhibitors have been shown to have a decreased incidence of GI toxicity, gastric ulceration and perforation are still possible and all classes of NSAIDs have the potential to induce renal failure or liver disease. In addition to the more traditional complications, the newer COX-2 selective NSAIDs may carry their own toxicity, such as cardiovascular complications.
Generally, NSAIDs are safe if the appropriate drug is used in the appropriate species and dosing guidelines are strictly followed. Careful patient and drug selection and monitoring will also help minimize the chance of complications. NSAIDs should be avoided in patients with preexisting renal disease or GI ulceration. They should also be used carefully in patients with preexisting liver disease, congestive heart failure, hypertension, ascites, bleeding disorders, or diseases associated with thromboembolism.