
A high-alert medication is a drug with increased risk for causing significant harm when incorrectly administered.1 These drugs include (but are not limited to) those with a narrow therapeutic index, and those that can cause severe adverse effects. Hazardous drugs, defined as drugs that are known or suspected to cause adverse health effects from exposures in the workplace, are found on the National Institute for Occupational Safety and Health (NIOSH) hazardous drug list.2,3
Knowing which drugs are considered high-alert or hazardous medications (see Author’s List of High-Alert & Error-Prone Medications), where to find more information, and how to identify these drugs is important.
Identifying High-Alert and Hazardous Medications
Institute for Safe Medication Practices & National Institute for Occupational Safety and Health
The Institute for Safe Medication Practices (ISMP) provides a list of high-alert medications, including epinephrine, transdermal opioids, and potassium chloride for injection concentrate.1 The NIOSH publishes a regularly updated list of hazardous medications, including drugs with a reproductive and/or neoplastic risk (eg, cyclosporine, misoprostol, methimazole, zonisamide, chemotherapeutic agents); some NIOSH-listed drugs are also found on the high-alert list.4
Look Alike Sound Alike (LASA) Drugs
Drugs with names that appear or sound alike can be mistaken for each other and therefore have heightened error potential. The ISMP provides a list of drug names that are often confused, some of which are also high-alert medications. This list can help clinic staff determine which drugs look and sound alike to avoid confusion that could lead to mistakes. For example, oxytetracycline could be mistakenly dispensed in place of oxytocin. These drugs should therefore be added to the clinic’s list of drugs with high error potential, separated on the shelf, labeled with tall man lettering (eg, oxyTOCIN vs oxyTETRACYCLINE), and dispensed using a 3-person check system; see Awareness & Prevention. Drugs with labeling or packaging that is similar to another drug also are examples of look-alike drugs that could contribute to medication errors.
Administration Mistakes
Simple mistakes with calculations, preparation, or administration can result in significant patient harm; therefore, careful attention to preparation, administration, distribution, and patient species is critical. For example, immediate-release (every 8 hours) and extended-release (every 12 hours) levetiracetam are administered at different frequencies, dispensing the incorrect levetiracetam product could result in harm because of failure to maintain appropriate therapeutic concentrations, possibly resulting in breakthrough seizures.
Some injection preparations (eg, potassium chloride for injection concentrate, insulin, oxytocin, epinephrine, anticoagulants, hypertonic saline) are also considered high-alert medications.1 For example, potassium chloride injection has a narrow safety index in veterinary patients and can interact with many other drugs when used in combination. Awareness of this drug’s maximum concentration and maximum rate of administration is important for patient safety.5
Awareness & Prevention
An updated high-alert/hazardous medication list should be available for clinic staff and should be reviewed and updated at least once per year. Auxiliary labels, tall man lettering for LASA drugs, a 3-person check system, limited access to high-alert/hazardous medications, and drug education can be helpful.
Auxiliary Labels
Auxiliary labels (Figure) can alert clinic staff to take extra precautions and encourage education of pet owners about these drugs. For hazardous medications that are antineoplastic and/or have reproductive risk, auxiliary labels can be added to the bottle as a reference tool for owners. Chemotherapuetics can be stored in a separate location in the clinic as a reminder that these drugs have distinctly different handling needs and auxiliary labels.
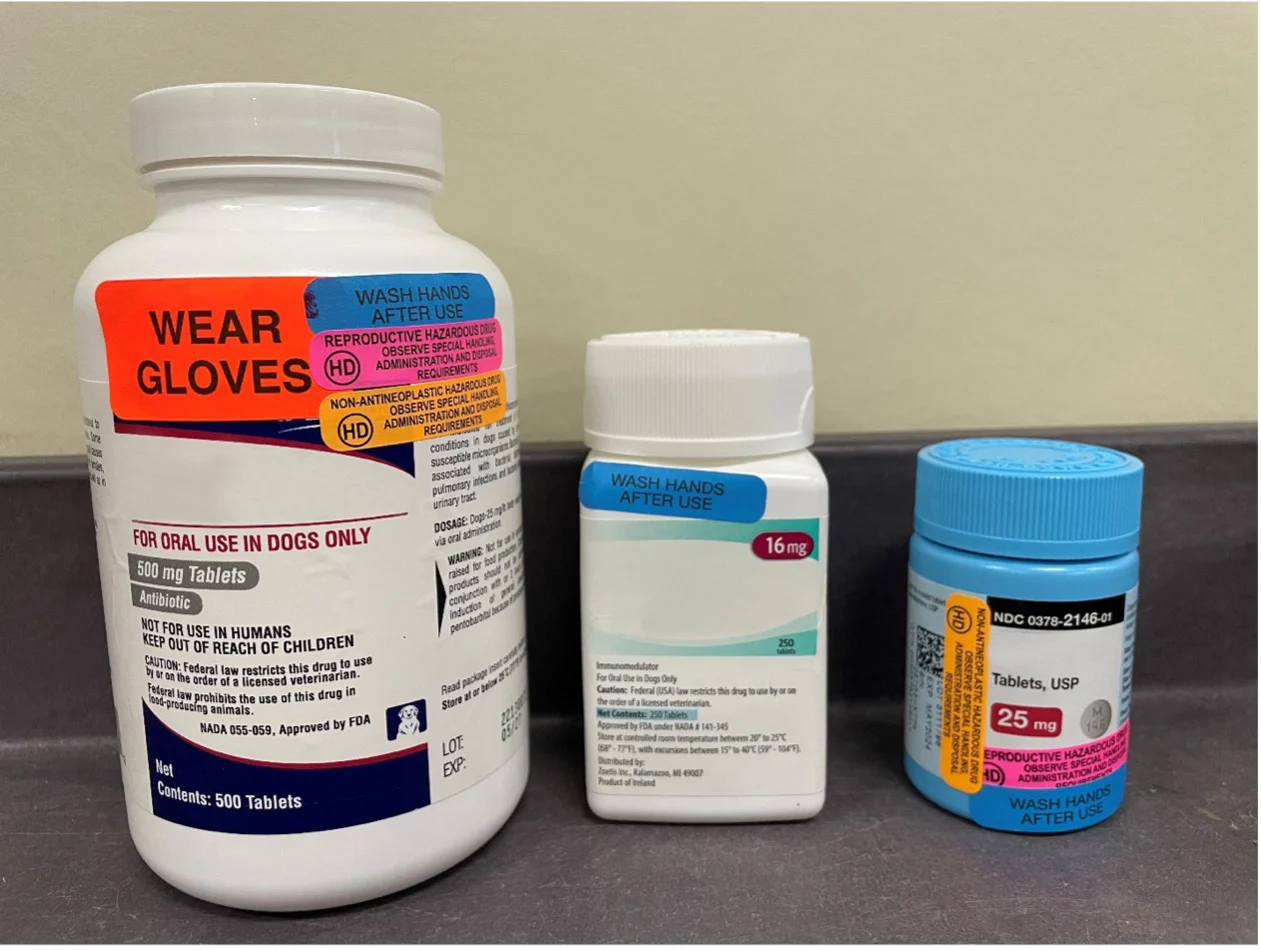
FIGURE Common auxiliary labels for high-alert medications
Tall Man Lettering
Tall man lettering is the use of uppercase letters to emphasize name differences (eg, trazODOne vs traMADol), which can help reduce errors related to drug name confusion. In a study, tall man lettering reduced name confusion in young adults, older adults, and healthcare practitioners.6 The ISMP provides a list of drugs in which tall man lettering is recommended,7 but this practice can also be incorporated for all drug labels.
Three-Person Check System
A 3-person check system can be implemented in the clinic, with different staff members performing each step of drug administration. A technician should first verify the dose, route, frequency, and duration of the prescription ordered by the clinician, a different technician or clinician should then fill the prescription, and finally, the initial clinician should check the prescription prior to administration.
Limited Access
Locking controlled drugs in a safe can limit access. Noncontrolled hazardous drugs (eg, toceranib) should be placed separate from the general dispensing area as a reminder that extra handling care is needed.
Education
A veterinary pharmacist or pharmacy continuing education course can help instruct clinic staff on the dangers of high-alert and hazardous medications and how these drugs should be handled.
Conclusion
Creating a high-alert/hazardous medication list can help prevent errors, increase pet owner and clinic staff education about the dangers of improper drug use, and potentially save lives. The list should be drawn from the ISMP and NIOSH high-alert and hazardous medication lists and additional drugs added, especially those that may have been previously used or filled improperly. Auxiliary labels, proper precautions, and clinic staff education should be implemented.