Managing Indwelling Urinary Catheters
Nicola Ackerman, BSc (Hons), RVN, CertSAN, CertVNECC, VTS (Nutrition), The Veterinary Hospital Group
Indications for urinary catheters include urinary obstruction, urinary trauma, voiding disorders, urine diversion during or after surgery, and the need to monitor urine production.1 Guidelines exist for urethral catheterization in humans,2 and general principles from these have been adapted for urethral catheter management in dogs and cats.
It is suggested that urethral catheters be placed in an aseptic manner, that antibiotics are not used prophylactically, and that a closed urinary-collection system is used to reduce the incidence of catheter-associated urinary tract infection.2-4 Alterations in the normal structure of the lower urinary tract, however, can make it easier for bacteria to adhere, grow, and create a probiofilm environment. Any event that causes trauma to the mucosal layer of the bladder and erodes the glycosaminoglycan layer (eg, placement of a urinary catheter, presence of stones or neoplasia) may cause a disruption in these natural defenses.5 Natural mechanisms in the bladder have been shown to clear induced UTIs within 2 to 3 days; however, the presence of a surgically placed foreign object in the bladder may cause animals to develop chronic UTIs with a concurrent biofilm on the foreign object in <24 hours.6
Placement and management of urinary catheters need to be conducted using aseptic technique, as introduction of bacteria can potentially occur and cause a nosocomial infection.
Placement and management of urinary catheters therefore need to be conducted using aseptic technique, as introduction of bacteria can potentially occur and cause a nosocomial infection. During catheter placement, all hair needs to be clipped and the site cleaned with dilute chlorhexidine or iodine, with no application of alcohol. Individual sterile sachets of lubricating gel should be used for the catheter placement, in addition to the use of sterile gloves. If no individual sachets are available, a new (unbroached) tube of lubricating gel should be used.
Urinary Catheter Care
Before handling of the urinary catheter or urinary-collection system, hands should always be washed (using the World Health Organization technique) and sterile gloves worn.7 In dogs, it is also recommended to rinse the prepuce or vulva with warmed antimicrobial solution at the correct dilutions at least every 4 hours.7 The area needs to be completely dried following each rinse. The external catheter and the collection line should also be wiped down with a 70% isopropyl alcohol swab.8 If there is gross contamination (eg, feces), the collection line should be replaced.
It is recommended to always use a closed urine-collection system to help quantify output and prevent leakage onto the skin or coat.
In male dogs, an abdominal dressing can be used to stop the dog from licking the area. White or light-colored dressings should be used to indicate any early evidence of any urine leakage through the bandage. In bitches, the urine-collection system can be secured to the hind leg.
Figure 1. Silicon cat catheter sutured in place with Luer adapter and urine-collection system.
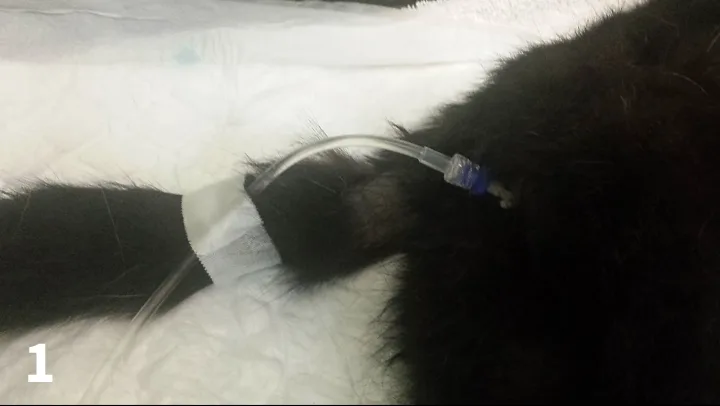
In male cats, taping the urine-collection system to the tail prevents direct pulling on the prepuce after the catheter has been sutured in place. If there has been a tail-pull injury, this should be avoided. In long-haired cats, reducing the length of the hair on the ventral aspect of the tail can prevent any hair from getting caught in the connection between the catheter and the collection system (Figure 1). In the female cat, catheterization is less frequently performed. The catheter can be sutured to the lip of the vulva, or a Foley catheter used with balloon and with the urine-collection system still secured to the tail (except in cases of tail-pull injury).
Figure 2. Closed urine-collection systems should be used in all cases.

It is recommended to always use a closed urine-collection system (Figure 2) to help quantify output and prevent leakage onto the skin or coat. Even when using a closed urine-collection system in cats, a litter tray should be provided not only for stools that may be passed but also to allow cats to display their natural elimination behavior of burying.
Management Protocol
Figure 3. Cat urinary catheter being flushed.
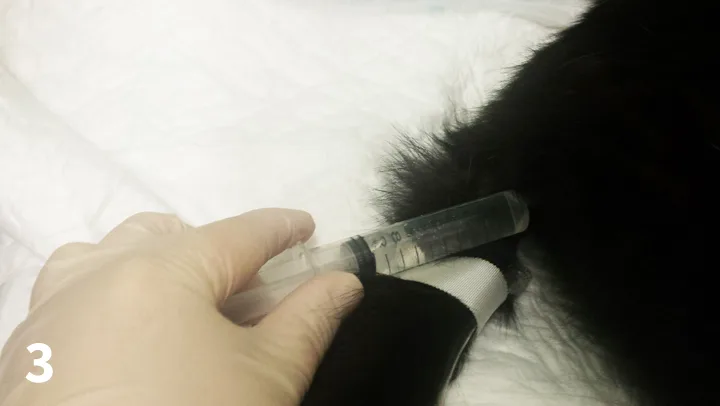
The urinary catheter should be checked for occlusions and adequate urine output every 4 hours.7 There is an increased likelihood of blockage in patients passing a large amount of debris in the urine, and therefore more regular checks may be required. This includes palpation of the bladder to ensure good drainage. If the bladder is firm and distended, there is a potential that the catheter is obstructed. The catheter can be slightly repositioned and the collection system checked for kinks.9 When there is a possibility for occlusions, 0.9% sterile saline solution can be used to flush the catheter (Figure 3). It is imperative that aseptic technique is used and that the flush is connected directly to the catheter rather than through the collection system, as, if bacteria have colonized the collection system, this can introduce the bacteria into the bladder. Likewise, urine should not be collected from the collection bag or urinary catheter for evaluation; the presence of bacteria in the collection system may not reflect true infection if bacteriuria, culture, and susceptibility of urine should be performed.7
If a volume of fluid(s) is required to keep the catheter patent, it needs to be subtracted from the volume of urine passed. Cats and dogs should be producing 1 to 2 mLs of urine per kg of body weight per hour when in a normovolemic, normotensive state with adequate kidney function (Figure 4). Hands should be washed and gloves worn at all times when attaching and disconnecting the urine-collection system.
Figure 4. Urine-collection systems allow accurate monitoring of urine output.

In addition to being checked for occlusions, animals with urinary catheters in place need to have their bladders palpated to ensure the organ does not become distended (ie, ensuring the catheter is draining properly). Gentle manual compression of the bladder should produce urine drainage. This can be done at the same time as flushing the catheter.
In some cases in which the catheter might be too small for the urethra, the animal might be able to pass urine around the catheter. It is essential that the catheter is neither too wide, which may exert pressure on the urethral mucosa, or too narrow, which may allow the patient to urinate around it and predispose to urinary leakage and urine scald on the adjacent skin. Of equal importance is the length. Ideally, the tip of the catheter should sit just within the lumen of the bladder. Shorter catheters will sit within the proximal urethra causing irritation and less efficient bladder drainage, whereas excessively long catheters may coil inside the bladder and cause irritation and possibly perforation.10 The patient’s bedding should be inspected for urine leakage and the patient’s coat checked for dampness whenever the patient is checked for urine output.
Figure 5. Fluid infusion pump.

Patients recovering from urethral obstruction will undergo postobstructive diuresis. Careful monitoring of the volume of urine produced vs the volume of fluid intake is vital. Use of a fluid pump (Figure 5) or burette can ensure the clinician or veterinary nurse can accurately calculate fluid input and output, which enables adjustment of supplemental fluid rate to prevent dehydration. Closed collection systems should be used with drainage available through a distal port rather than the catheter connection site.7 This evacuation port should be wiped down with dilute chlorhexidine after each use.
Urinary catheter management is an important aspect of nursing care. Prevention of nosocomial infections and urine scalding is exceptionally important; these can be avoided with good nursing management of the patient.