Image Gallery: Lymph Node Cytology
Maxey Wellman, DVM, MS, PhD, DACVP (Clinical Pathology), The Ohio State University
M. Judith Radin, DVM, PhD, DACVP (Clinical Pathology), The Ohio State University
Lymphadenopathy (ie, lymphadenomegaly) can be caused by lymphoid and plasma cell hyperplasia, inflammation, or lymphoid and metastatic neoplasia. Cytology of lymph node aspirates is easily performed in general practice while being relatively noninvasive and inexpensive. Depending on clinician experience, results can provide a quick, accurate diagnosis when coupled with clinical history and physical findings.
Lymph Node Aspiration Procedure
Choosing a sample site
If multiple lymph nodes are enlarged, sampling of mandibular lymph nodes should be avoided because they often are hyperplastic and mildly inflamed. Other peripheral lymph nodes that are easily sampled include popliteal and prescapular lymph nodes. Extremely enlarged lymph nodes also should be avoided because necrosis or hemorrhage may preclude adequate sampling and evaluation.
Sampling technique
Lymph node samples should be collected with a 21- or 25-gauge needle using an aspiration or nonaspiration technique. For the aspiration technique, a 21-25-gauge needle coupled to a 12-20 ml syringe is used, whereas for the non-aspiration technique, only the needle is used to prick the lymphoid tissue, using the cutting action of the needle to collect the sample. The needle can then be attached to a syringe prior to making the smears. If only a small amount of material has been collected, the needle is detached, air is aspirated into the syringe, the needle is replaced, and a small amount of material is carefully expelled onto several clean glass slides.
Redirecting the needle to several locations within the lymph node is recommended to ensure that a representative aspirate has been obtained. Only a small amount of material is needed.
Sample handling
After capturing the sample, the contents should be sprayed on to a clean slide using a clean, empty syringe. A spreader slide should be used to gently disperse the cells, creating a thin monolayer of cells.
Common errors during slide preparation include:
Failure to disperse the cells on the slide: results in smears that are too thick, and
Applying too much pressure with the spreader slide, resulting in broken cells. Broken cells may not be reliably evaluated.
Smears should be clearly labeled with pencil or a marker specifically designated for sample labeling. Smears should be dried in ambient air before staining.
Slides should not be stored in a refrigerator or exposed to formalin because water condensation from refrigeration can lyse cells and formalin fumes can prohibit adequate staining.
Slide Staining
Because additional staining, immunophenotyping by flow cytometry, and clonality testing sometimes can be helpful to detect causative agents, determine cell lineage, or support a diagnosis of neoplasia, saving several unstained slides is recommended.
Romanowsky-type stains (eg, Wright's, Wright-Giemsa, Diff-Quik or comparable commercial quick stain) provide good color contrast and acceptable cytoplasmic and nuclear detail, plus can stain most infectious agents.
If slides are being sent to a reference laboratory for interpretation, patient signalment, relevant clinical and laboratory findings, and the degree and extent of lymph node enlargement should be described in detail.
Image Gallery: Lymph Node Cytology
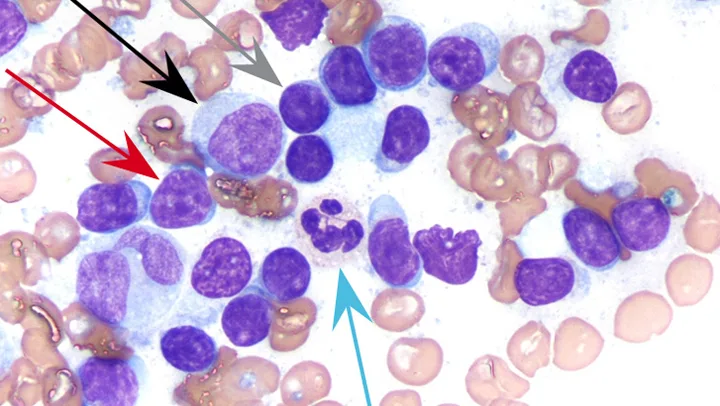
FIGURE 1 Normal lymph node.
Most (85%-95%) of the cells (gray arrow) are small lymphocytes (ie, smaller than a neutrophil [blue arrow]), with round nuclei, condensed chromatin; inconspicuous nucleoli; and scant, pale cytoplasm, resulting in a high nuclear:cytoplasmic (N:C) ratio. Occasional intermediate lymphocytes (red arrow), which are similar in size to neutrophils, have less condensed chromatin and slightly more cytoplasm. Occasional large lymphocytes, sometimes called lymphoblasts (black arrow), are larger than neutrophils, and have finely stippled chromatin and nucleoli, along with moderate amounts of basophilic cytoplasm. Numerous RBCs are consistent with blood contamination. (Wright-Giemsa stain, magnification 1000×)