Lameness & Osteomyelitis in a Cat
Lauren Chapman, DVM, VCA North Coast Animal Hospital, Encinitas, California
Ryan Taggart, DVM, MS, DACVS-SA, Adelaide Veterinary Specialist & Referral Centre, Adelaide, Australia
History
A skeletally mature (age unknown), 8.6-lb (3.9-kg) spayed domestic shorthair cat was evaluated for acutely worsening left pelvic limb lameness. Mild lameness had been observed for approximately 4 weeks before presentation. The patient was not receiving any medications and had been adopted in New Mexico 4 years prior. The owner reported no travel history since adoption; however, limited prior medical records indicated that the patient had been vaccinated in Northern California prior to adoption.
Physical Examination
The principal physical examination finding was a firm, painful, circumferential swelling of the left proximal metatarsal/tarsal region accompanied by a nonweight-bearing lameness on that limb. The remainder of the physical examination was unremarkable. Rectal temperature was 101.8°F (38.8°C), heart rate was 200 bpm, and respiratory rate was 36 breaths/min with no increased respiratory effort observed.
Diagnostics
Serum chemistry profile, urinalysis, and CBC were unremarkable, and the patient was negative for FIV antibodies and FeLV antigen.
Radiographs of the left proximal metatarsal/tarsal region revealed multiple foci of osteolysis within the distal tarsal and proximal metatarsal bones, as well as marked soft tissue swelling of the area (Figure 1). Periosteal reaction was noted on the dorsal and plantar surfaces of the distal tarsal and proximal metatarsal bones.
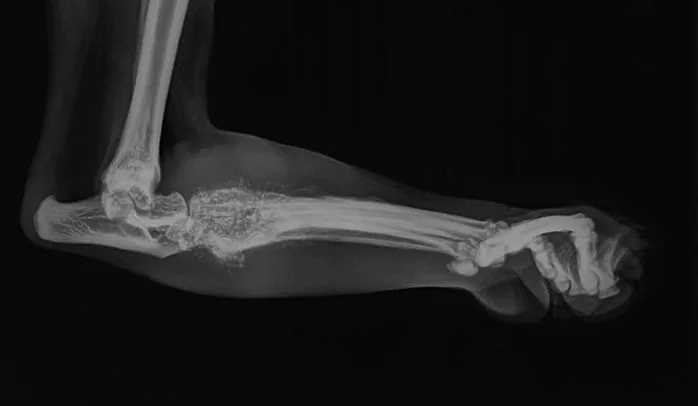
Multiple foci of osteolysis and surrounding marked soft tissue swelling in the distal tarsal and proximal metatarsal bones
Radiographs of the thorax revealed a diffuse, finely granular interstitial pulmonary infiltrate with prominent bronchial markings with indistinct nodules in the middle and caudal lung lobes (Figure 2).
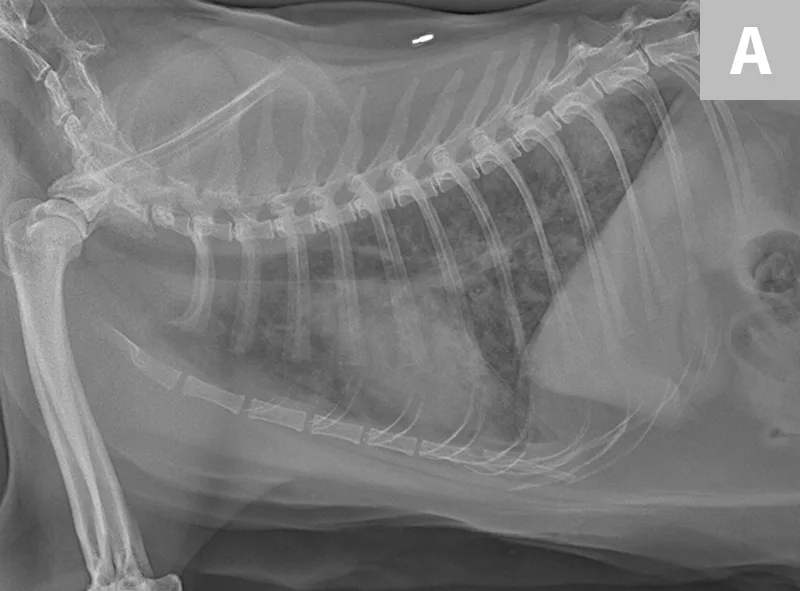
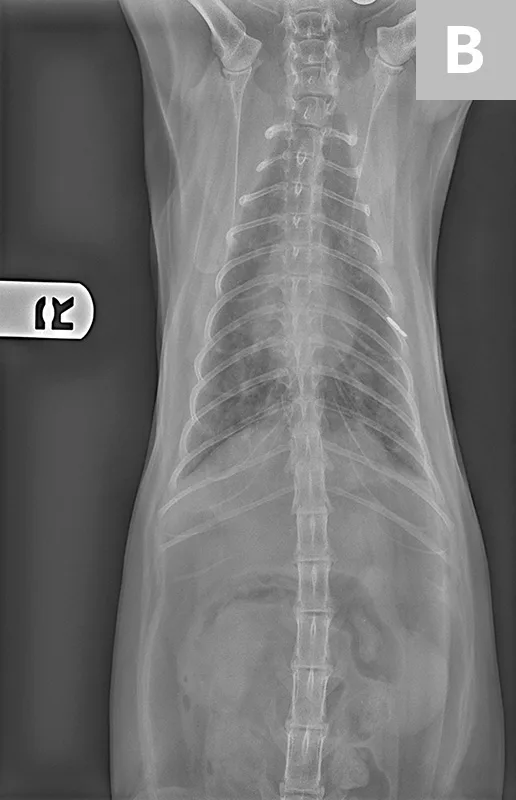
Diffuse, finely granular interstitial pulmonary infiltrate (A) with prominent bronchial markings with indistinct nodules in the middle and caudal lung lobes (B)
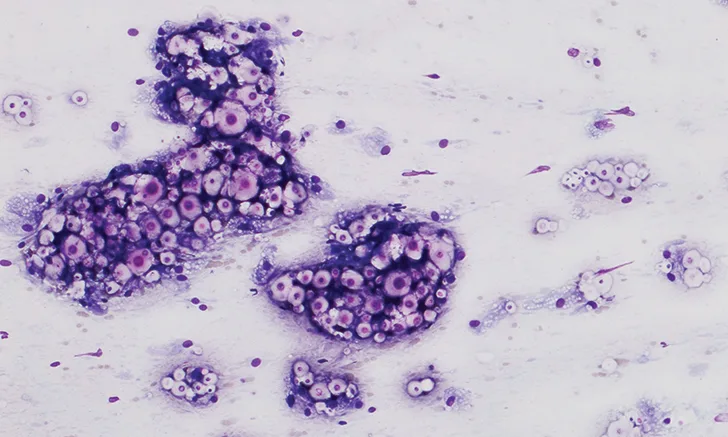
A fine-needle aspirate was collected from the proximal metatarsal/tarsal swelling (Figure 3). Cytologic evaluation revealed a granulomatous inflammatory infiltrate and abundant capsulated yeast organisms demonstrating occasional narrow-based budding. The morphology of these organisms was consistent with Cryptococcus spp infection.
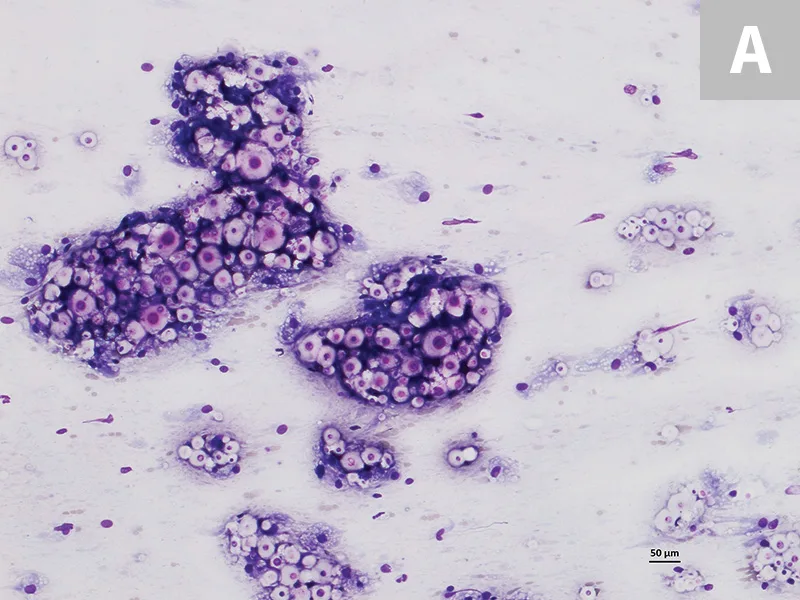
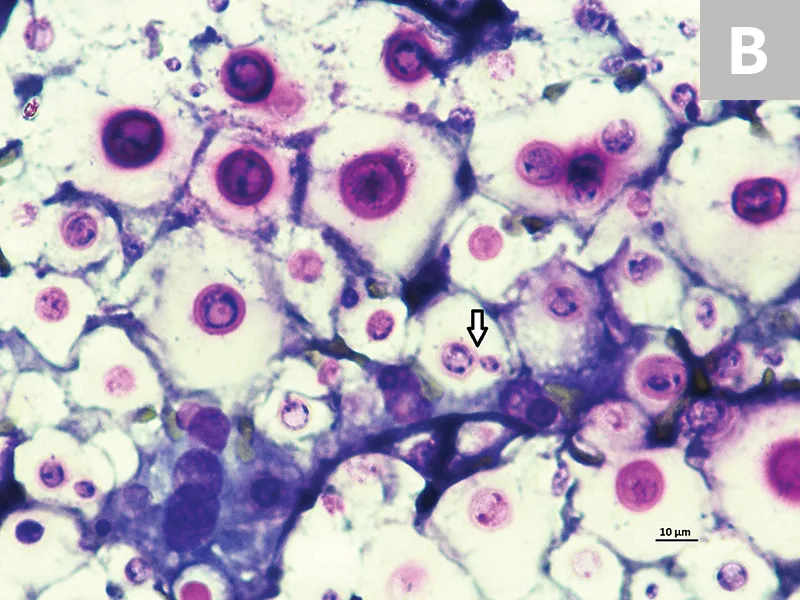
Cytology of the fine-needle aspirate from swollen tarsus with numerous Cryptococcus spp yeast organisms featuring thick clear capsules (A). Narrow-based budding is displayed by some of the organisms (B; arrow). 100× and 500× magnification, Wright-Giemsa
A Cryptococcus spp antigen agglutination test was positive (titer 1:3688). Because of the uncommon presentation of fungal osteomyelitis, biopsies of the tarsal swelling were submitted for histopathologic analysis and fungal identification by genetic sequencing. Histopathologic analysis confirmed the organisms were consistent with Cryptococcus spp and described severe, focally extensive, granulomatous osteomyelitis and cellulitis with intralesional encapsulated yeast organisms. DNA-based analysis matched that of Cryptococcus gattii type V, molecular type VGIII.
Treatment & Outcome
Treatment was initiated with fluconazole (12.8 mg/kg PO q12h) and buprenorphine (0.015 mg/kg transmucosal q8h) for pain.1,2 Fluconazole was used for about one month. Following a discussion with a veterinary mycosis specialist, treatment was changed to itraconazole (12.8 mg/kg PO q12h; later reduced to 9 mg/kg PO q12h) and amphotericin B (0.55 mg/kg as a diluted [in 350 mL of 0.45% NaCl with 2.5% dextrose] twice-weekly SC infusion) based on the results of diagnostic testing that further classified the Cryptococcus spp strain.
Clinical signs resolved within 2 months and did not return. A total of 20 doses of amphotericin B were administered to reach a cumulative dose of approximately 10 mg/kg. Hepatic and renal parameters were monitored. Renal values remained within the reference interval, but a mildly elevated alanine aminotransferase persisted (162-249 U/L [2.71 - 4.16 μkat/L]; reference range, 10-100 U/L [0.17 - 1.67 μkat/L]). Based on intolerance (ie, inappetance, vomiting) to the initial dose of itraconazole, the dose was reduced to 8.5 mg/kg PO q12h, and treatment was continued for approximately 2 years after initiating therapy until the patient was seronegative on 2 occasions one month apart. At the final follow-up, the patient appeared healthy with no signs of illness. Lengthy, possibly lifelong, treatment may be required for some patients.3 Even when clinical signs resolve, relapse is still possible.4 A better prognosis has been observed in animals treated early or those with only localized disease without CNS or systemic involvement.5
Discussion
C gattii was long considered an organism of tropical and subtropical climates. However, C gattii-endemic areas now include western Canada and the Pacific Northwest region of the United States.6
Unlike infection with other Cryptococcus spp organisms, Cryptococcus gattii infection tends to occur in immunocompetent individuals.3 In cats, infection typically occurs after basidiospore entry into the patient from the environment via inhalation and colonization of the nasal cavity, followed by tissue invasion of structures in the sinonasal cavity. Extension occasionally occurs into the CNS, oral cavity, and/or orbit by penetration of bones surrounding the sinonasal cavity.
This patient’s presentation is more consistent with human infection, in which the lung is typically the primary site of infection, with subsequent hematogenous dissemination to brain, bone, skin, and/or other tissues.6 C gattii is not considered to be zoonotic, except in immunocompromised humans.4 Rather, humans and animals acquire the organisms from the same environmental sources. Animals may serve as important sentinels, which indicates the potential for human exposure from the environment.5 The incubation period between exposure and clinical signs is variable and reported to be 1 to 12 months or longer.5
Young to middle-aged cats are most often diagnosed with cryptococcosis, but all ages can be affected.4 Distinguishing among species of Cryptococcus has become important in epidemiologic studies, but there is no difference between the clinical presentations of infections caused by different members of the C neoformans/gattii species complex.4
Cats are reportedly 6 times more likely than dogs and 3 times more likely than horses to become clinically affected.4 Lesions are most often noted in the nasal, maxillary, or frontal regions of the head.3 Fungal granulomas or lesions involving the lymph nodes and skin of the head and neck are common.5 CNS and/or ocular involvement is suspected if blindness, retinal (chorioretinitis), or optic disc lesions accompany a diagnosis.4 Almost any organ system, including the lungs, kidneys, bone, and periarticular tissues, can be affected.4 Treatment typically consists of antifungal therapy, with close monitoring of liver and renal values.