Intestinal Endoscopic Biopsy Techniques
The availability and use of flexible endoscopy has led to a marked increase in diagnostic procedures involving visualization and biopsy of the small and large intestines in companion animals.
Endoscopy allows rapid, minimally invasive examination of mucosal surfaces and permits procurement of tissues for histologic and cytologic examination. One of the keys to success in endoscopic detection of GI mucosal disease is proper biopsy technique.1
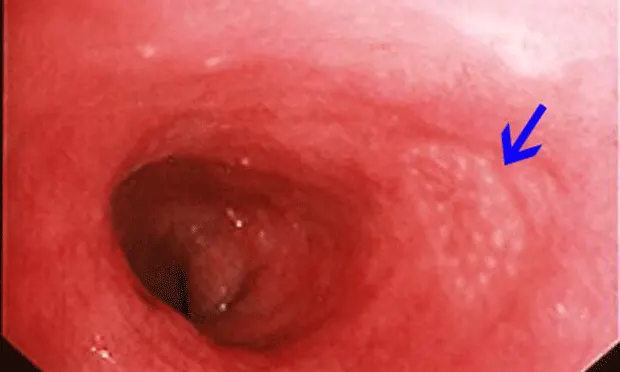
Normal Endoscopic Appearance of IntestinesCompared to other alimentary tract organs, the duodenal mucosa has a more granular texture, due to its prominent villous architecture. Duodenal and jejunal mucosa color varies from pale pink to tan in animals having recent biliary secretion. Submucosal lymphoid aggregates appear as oval, slightly depressed mucosal structures present along the descending duodenum (in dogs) and should not be interpreted as mucosal lesions. Other normal mucosal structures include the major duodenal papillae (present in the dog and cat) and minor duodenal papillae (present only in the dog) (Figure 1).
Gastrointestinal Endoscopy in Dogs
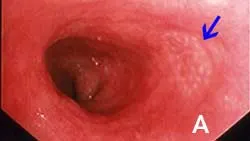
(Figures 1A and 1B: Endoscopic appearance of the normal proximal duodenum in a dog. A lymphoid aggregate (A, arrow) is clearly visible along the lateral mucosal wall. This structure should not be biopsied as such samples confound histologic determination of lymphocytic enteritis.
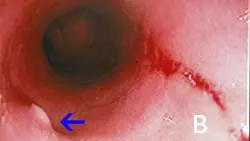
The major duodenal papilla (B, arrow) and a linear mucosal erosion caused by passage of the endoscope through the cranial duodenal flexure are shown in B.)
Normal colonic mucosa is pale pink, smooth, and glistening. Submucosal blood vessels are readily observed with adequate insufflation in dogs. Lymphoid aggregates, 2 to 3 mm in diameter and umbilicated, are most common within the rectum and cecum. Visual inspection of the cecum and ileocolic sphincter should be performed in all patients undergoing full colonoscopy. Parasitism, cecal inversion, ileocolic intussusception, inflammatory bowel disease, and neoplasia may cause mucosal lesions in this region.
Biopsy Instrumentation
Appropriate biopsy instrumentation includes flexible biopsy forceps. Several styles are available, including those with smooth or serrated cups and with/without a central spike or bayonet (Figure 2).1 Personal preference and operator experience determine the selection of forceps type; serrated-cup forceps without a central spike are often preferred since they cause fewer artifacts to tissue specimens.

(Figure 2: Smooth-cup forceps with a central stylet, top, serrated-cup forceps with a central stylet, middle, and serrated-cup forceps without a stylet, bottom)
The diameter of the endoscope’s accessory channel will dictate what size forceps to use. A channel size of 2.8 to 3 mm facilitates the use of a large forceps, which procures larger tissue samples for mucosal biopsy. However, forceps passed through smaller diameter endoscopes still may yield excellent quality biopsies for diagnostic purposes.2
STEP BY STEP ENDOSCOPIC BIOPSY: SMALL INTESTINE3
IndicationsEnteroscopy is indicated in patients with clinical signs of small intestinal disease, including:
Chronic small bowel diarrhea
Weight loss
Alterations in appetite (often anorexia)
Vomiting
Melena
Specific small intestinal diseases diagnosed via enteroscopy include inflammatory bowel disease (IBD), intestinal neoplasia, duodenal ulcer/erosions, lymphangiectasia, and GI histoplasmosis. Enteroscopy is particularly useful in patients with protein-losing enteropathy in which debilitation and hypoalbuminemia may increase the risk for surgical dehiscence.
Patient PreparationWithhold food for 12 to 18 hours for upper small bowel examination. Retrograde ileoscopy will require more extensive patient preparation (see Step 2, page 15).
Abnormal FindingsAlterations in mucosal texture (increased granularity), friability, and hyperemia are commonly observed, and these abnormalities are often associated with mucosal inflammation seen with IBD and intestinal neoplasia. Ulcers (uncommon) and erosions (more common) are characteristic of inflammatory and neoplastic lesions. A milky-white mucosal appearance from distended villi or milky exudate within the lumen from ruptured lymphatics may be seen in dogs with intestinal lymphangiectasia.
Recommendations & TechniqueDuodenal/jejunal biopsies should always be obtained regardless of mucosal appearance. Duodenal tissue is normally quite friable and good technique is essential. Obtain 10 to 15 good-quality specimens. Focal mucosal lesions (eg, erosions or granular areas) are biopsied directly since these abnormalities often correlate to histopathologic lesions.
The best biopsies are obtained by directing the instrument at a 90° angle (perpendicular) to the mucosal surface at flexure locations (A). (Note the numerous white spots along the mucosa, which are areas of light reflection, not lymphangiectasia.) Flexure sites facilitate targeted, deep mucosal tissue purchases while reducing the tendency of the forceps cups to slide along the superficial mucosa. Tissue biopsies procured at nonflexure sites will require directing the endoscope tip (and biopsy forceps) obliquely toward the mucosa.
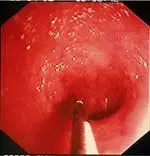
If the patient is large, use of a “long-distance” biopsy technique may be warranted. The biopsy forceps is passed as far as possible down the lumen until resistance is met. Usually the forceps cups are beyond the operator’s view distally (B). Once resistance is encountered, the forceps are retracted slightly (to open up the jaws) then readvanced firmly against the mucosa where a tissue specimen is obtained (C).
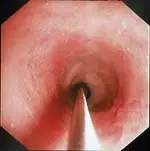
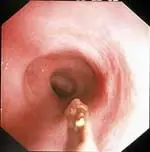
The disadvantages of this technique are its “blinded” approach and the tendency to biopsy repeatedly in the same site. Mucosal perforation is a potential but extremely unlikely complication of this procedure. The technique is often used and quite successful.
STEP BY STEP ENDOSCOPIC BIOPSY: LARGE INTESTINE4
IndicationsColonoscopy is indicated in patients with clinical signs of chronic colonic disease, including:
Large bowel diarrhea
Tenesmus
Dyschezia
Hematochezia
Mucoid feces
Colonoscopy is particularly useful in the diagnosis of IBD (lymphocyticplasmacytic colitis), colonic neoplasia, and rectal masses in both dogs and cats.
Patient PreparationFood should be withheld for 18 to 24 hours. While longer periods (24–36 hours) may result in less retained feces, this time period is often impractical for many clients. In dogs, I prefer to administer 2 doses (20 mL/kg given 4–6 hours apart, orally) of a colonic electrolyte lavage solution (GoLYTELY; nulytely.com). It has been my experience that these lower doses provide excellent mucosal cleansing while higher doses (40–50 mL/kg) may cause vomiting in some patients. The cleansing solution may be administered either by orogastric intubation with a stomach tube or by offering the solution free-choice in a water bowl. A warm-water enema (20 mL/kg) can then be administered the morning of the procedure to both dogs and cats.
Abnormal FindingsAbnormal findings are similar to those found during enteroscopy, including increased mucosal granularity, increased friability, and the presence of ulcer/erosions. If visualization of submucosal vessels is obscured, it is a significant finding and may be caused by mucosal edema, accumulation of exudate (blood, mucus, necrotic debris), or infiltration by inflammatory or neoplastic cells. Rectal masses and colonic nematodes (Trichuris vulpis) are less common observations.
Recommendations & TechniqueColonic biopsies should always be obtained regardless of mucosal appearance. Flexible endoscopy is preferable since examination and biopsy of the transverse and ascending colons may also be performed. Focal mass lesions or mucosal lesions of increased granularity and/or erosions are biopsied directly. In the absence of gross mucosal abnormalities, I obtain 3 to 4 biopsy specimens from each colonic region (eg, ascending, transverse, and descending colons).
Retrograde ileoscopy should be performed as part of routine lower GI endoscopic examination in large dogs; it is contraindicated in small dogs and cats because it may cause too much trauma. This procedure is useful when an insufficient quantity or poor-quality duodenal biopsy samples have been obtained. It necessitates thorough colonic cleansing and advancement of the endoscope tip through the ileocecal sphincter in dogs (A).
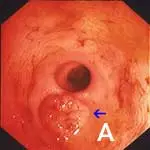
Note the prominent and protuberant ileocolic valve (arrow); focal areas of hemorrhage are due to a previous mucosal biopsy. Also note that the ileal mucosa has an identical appearance to that of the proximal duodenum (B).
Alternatively, “blind” biopsies may be procured by passing the biopsy forceps through the ileocecal orifice in cats. Potential risks include repeated sampling of similar areas of the ileal mucosa and intestinal perforation (rare). Ileal biopsies may be particularly useful for diagnosis of lymphosarcoma in cats.5
BIOPSY HANDLING CONSIDERATIONSSpecimens should be carefully removed from forceps cups with a 25-gauge needle, being careful to position the specimen muscularis side down on a biopsy sponge. Note that biopsy artifacts, such as superficial mucosa and crush artifacts, are inherent in most biopsy procedures regardless of operator experience and technique (Figures 3 and 4).1
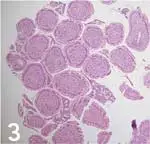
(Figure 3: This biopsy sample shows duodenal villous tips (eg, “villous slaw”) devoid of subvillous lamina propria and crypts. This artifact may occur when samples are quickly procured using poor biopsy technique or can be caused by improper specimen processing by the pathology service. In this latter situation, tissue sections are cut perpendicular to the villous axis, which makes it impossible to obtain full-thickness mucosal samples for microscopic review.)
(Figure 4: This figure shows significant crush artifact (circle) of the lamina propria, which obscures mucosal architectural features.)
Obtaining at least 8 biopsy specimens per organ will result in the greatest diagnostic accuracy for detecting most mucosal diseases affecting the small intestine.2 Studies evaluating the optimum number of endoscopic biopsies required for diagnosis of colonic diseases have not been performed.
Finally, to minimize misdiagnosis, use an experienced histopathology laboratory staffed by pathologists who are experienced in reading and interpreting endoscopic tissue specimens.6
For related articles, please see the following:Gastrointestinal Signs in a CatPercutaneous Endoscopic Gastrostomy Tube PlacementLaparoscopic Liver Biopsy
INTESTINAL ENDOSCOPIC BIOPSY TECHNIQUES • Albert E. Jergens
References1. Endoscopic biopsy specimen collection and histopathologic considerations. Jergens AE, Moore FM. In Tams TR (ed): Small Animal Endoscopy —St. Louis: Mosby, 1999, pp 323-340.2. Quality of tissue specimens obtained endoscopically from the duodenum of dogs and cats. Willard MD, Lovering SL, Cohen ND, et al. JAVMA 219:474-479, 2001.
Endoscopic examination of the small intestine. Tams TR. In Tams TR (ed): Small Animal Endoscopy—Philadelphia: Mosby, 1999, pp 173-216.
Colonoscopy. Willard MD. In Tams TR (ed): Small Animal Endoscopy—Philadelphia: Mosby, 1999, pp 217-245.5. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. Evans SE, Bonczynski JJ, Broussard JD, et al. JAVMA 229:1447-1450, 2006.6. Interobserver variation among histopathologic evaluations of intestinal tissues from dogs and cats. Willard MD, Jergens AE, Duncan RB, et al. JAVMA 220:1177-1182, 2002.7. Cytologic examination of exfoliative specimens obtained during endoscopy for diagnosis of gastrointestinal tract disease in dogs and cats. Jergens AE, Andreasen CB, Hagemoser WA, et al. JAVMA 213:1755-1759, 1998.