Overview
Hyperadrenocorticism (HAC) is caused by excess circulating cortisol or other corticosteroid hormones.
Epidemiology
Signalment
Species
Pituitary-dependent hyperadrenocorticism (PDH) occurs more frequently in smaller dogs; 75% of dogs with PDH weigh <44 lb (20 kg).1-3
Breed Predisposition
Poodles, dachshunds, boxers, and terrier breeds may have a greater risk for PDH.4
Age
Endogenous HAC occurs in middle-aged to older dogs.
Although the reported age range is 6 months to 20 years, almost all dogs with HAC are >6 years of age (mean, 11 years).4
Sex
There is no sex predisposition for PDH.
Female dogs may have increased risk for adrenal-dependent hyperadrenocorticism (ADH).1
Causes & Risk Factors
Endogenous HAC is either caused by an ACTH-secreting pituitary tumor (≈85% of dogs) or a benign or malignant adrenal tumor (≈15% of dogs).4
Rarely, endogenous HAC may be caused by ectopic ACTH secretion from a nonpituitary tumor or food-dependent hypercortisolemia.4-6
Iatrogenic HAC is caused by administration of exogenous glucocorticoids of any form.
History
Polyuria (PU) and polydipsia (PD) are most common (≈90% of dogs with HAC).4,7
Polyphagia, weight gain, and panting affect 50% to 90% of dogs.
Alopecia occurs in ≈60% to 75% of affected dogs.4,7
Lethargy may be associated with muscle weakness and inability to rise.
Behavioral changes (eg, disorientation, pacing, anorexia) are possible in dogs with a pituitary macroadenoma.
Physical Examination
On physical examination, a distended or pendulous abdomen (ie, potbellied appearance) is common (Figure 1) and caused by abdominal muscular weakness, hepatomegaly, redistribution of fat, or urinary bladder distension. Muscle wasting may be present; pseudomyotonia is uncommon and results in a stiff pelvic limb gait with a straight-leg appearance. Stupor or mental dullness may be noted in dogs with pituitary macroadenomas.

FIGURE 1 Typical potbellied appearance and generalized hair thinning in a dog with PDH
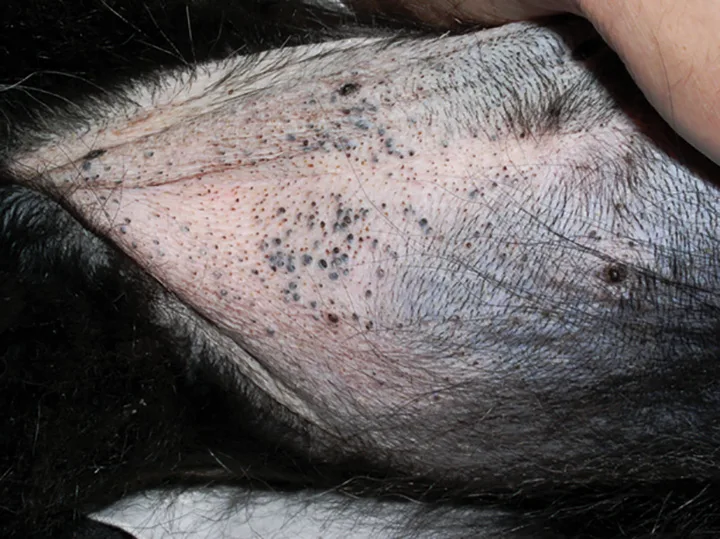
Dermatologic findings include alopecia, thinning of the skin, pyoderma, comedones, hyperpigmentation, and, less commonly, calcinosis cutis (Figures 2-6).

Figure 2 Close up view of the alopecia of the distal limb and foot.
Differential Diagnoses
Other causes of PU/PD (eg, renal disease, hepatic disease, diuretics, psychogenic PD)
Other causes of dermatologic findings (eg, hypothyroidism, atopy)
Other causes of laboratory findings (eg, primary hepatobiliary disease)
Diagnostics & Diagnostic Findings
Definitive Diagnosis
HAC diagnosis depends on consistent clinical signs and laboratory findings, exclusion of exogenous administration of glucocorticoids, and adrenal function testing.
Adrenal function tests for HAC can be separated into screening and differentiating tests.
Screening Tests
Screening tests are used to confirm HAC; results can be considered consistent or not consistent with diagnosis.
If results are not consistent with HAC but clinical suspicion is high, a different screening test should be pursued or testing repeated in 3 to 6 months if initial clinical signs are mild.
Testing should be postponed if significant concurrent illness is present due to increased risk for false positive results.
Urine cortisol:creatinine ratio has good sensitivity (90%-100%) but poor specificity (20%-40%).4,8-11
This ratio can be used for screening when clinical suspicion is low but HAC is considered as a differential.
A first-morning urine sample collected 2 to 3 days or more after the visit to the clinic or other stressful event is recommended.
A low-dose dexamethasone suppression (LDDS) test has a reported sensitivity of 85% to 100% and specificity of 44% to 73%.4,11-15
After obtaining blood for baseline cortisol, dexamethasone (0.01-0.015 mg/kg IV) should be administered and additional blood samples obtained after 4 and 8 hours for cortisol measurement.
LDDS is considered the screening test of choice in dogs with endogenous HAC.8 The author prefers this test for all dogs with suspected ADH.
An ACTH stimulation test has a reported sensitivity of 60% to 85% and specificity of 60% to 90%.4,11,14,16-18
ACTH stimulation is the only test that can differentiate endogenous and iatrogenic HAC.
This test cannot differentiate PDH and ADH.
After obtaining blood for baseline cortisol, synthetic ACTH (5 micrograms/kg IV or IM) should be administered and an additional blood sample obtained after 1 hour for post-ACTH cortisol measurement.
Differentiating Tests
Differentiating tests are used to determine whether HAC is caused by PDH or ADH.
Differentiating tests should only be used after diagnosis has been established with screening tests.
Endogenous ACTH measurement should be low in dogs with ADH and high in dogs with PDH.
Because of the pulsatile release of ACTH, dogs with PDH may have ACTH concentrations within range.
ACTH is degraded rapidly by plasma proteases. Appropriate sample handling (as per instructions from the reference laboratory) is necessary for accurate results.
The LDDS test is unique in that it can serve as a screening and differentiating test if the 8-hour cortisol measurement is higher than the laboratory reference.
If cortisol at 4 hours is <50% of the basal cortisol, the test is consistent with PDH.
If cortisol at 8 hours is <50% of the basal cortisol but greater than the laboratory cutoff for normal suppression, the test may also be consistent with PDH.
Lack of suppression could be caused by PDH or ADH.13
A high-dose dexamethasone suppression test (0.1 mg/kg IV) can be used if there is no suppression on the LDDS test to indicate PDH as above.
If cortisol at 4 or 8 hours is <50% of the basal cortisol, suppression has occurred, and the test is consistent with PDH.
Suppression is not seen in ≈25% of dogs with PDH on either LDDS or high-dose dexamethasone suppression tests, and suppression does not occur in most dogs with ADH.13
Additional Diagnostic Findings
Laboratory Results
Common CBC findings include a stress leukogram.
Thrombocytosis and, less commonly, mild erythrocytosis may be noted.
On serum chemistry profile, increased ALP is seen in most (≈85%-90%) but not all dogs with HAC.
ALT activity may be mildly increased.
Other findings include hyperlipidemia, fasting hyperglycemia, decreased BUN, and hypophosphatemia.
On urinalysis, urine specific gravity is commonly <1.020, but urine concentration can be variable.
Proteinuria is common.
Approximately 10% to 50% of dogs are reported to have either bacteriuria or a positive urine culture; most cases are subclinical bacteriuria, with <5% of positive cultures having signs of cystitis.4,19,20
Pyuria, stranguria, and hematuria may not be present because of the anti-inflammatory effects of cortisol.1,7,9
A urine culture should be performed if clinical bacteriuria is suspected.
In the absence of other evidence (eg, pyelonephritis), these dogs should be monitored via urinalysis and clinical signs to determine whether there is true infection once HAC is controlled.
Imaging
Thoracic radiography
Mineralization of the trachea or bronchi is common.
Radiographs should be evaluated for evidence of metastasis in dogs with ADH.
Pulmonary thromboembolism is uncommon in dogs with HAC but may be seen.
Abdominal radiography
Hepatomegaly is seen in 80% to 90% of dogs.
Approximately 50% of dogs with ADH have adrenal glandular mineralization.
Calcium-containing uroliths may be found.21-23
The author prefers abdominal radiography to screen for uroliths, as ultrasonography may miss stones in the ureters or urethra.
Abdominal ultrasonography
The author prefers abdominal ultrasonography to help differentiate PDH and ADH.
With PDH, adrenal glands are typically symmetrical and may be normal in size or enlarged.
With ADH, enlargement and loss of shape of 1 adrenal gland with atrophy of the contralateral gland is common.
The contralateral gland size is normal in some cases.
Ultrasonography can be used to evaluate for vascular invasion or evidence of metastasis.
CT/MRI
The pituitary can be imaged with CT and MRI for evidence of a tumor.
MRI is more sensitive than CT.
Tumors >1 cm are more readily seen on CT.
CT and MRI can be used to assess adrenal tumors and look for evidence of vascular invasion or metastasis.
The author typically recommends CT or MRI of the pituitary only when other tests are unable to differentiate PDH from ADH or if neurologic signs (eg, mental dullness, anorexia, circling) of a macroadenoma are present.
Advanced imaging should be performed if hypophysectomy or radiation is being considered.
Treatment
Treatment for HAC should only be considered once a diagnosis has been established.
The author does not recommend trial therapy to confirm diagnosis, but this may be considered in dogs with compatible clinical signs and inconclusive/borderline testing.
Whether treatment is indicated should be determined at the time of diagnosis.
Dogs that are subclinical or have only mild signs (eg, increased ALP, hepatomegaly, mild alopecia) likely do not need treatment but can be monitored for progression of signs.
Dogs with significant PU/PD, dermatologic manifestations, or recurrent infections should be treated.
Hypertension or proteinuria may indicate treatment of HAC; anecdotally these comorbidities require specific treatment in addition to HAC treatment.
Concurrent Canine Hyperadrenocorticism & Diabetes Mellitus
Hyperadrenocorticism and diabetes mellitus can be associated in dogs, but what is the nature of their connection? Learn how to keep your HAC patients from also becoming your diabetic patients.
Medical
Medical management is most commonly used for PDH but can be used for ADH if adrenalectomy is not an option.
Trilostane and mitotane are used most commonly and have similar efficacy for PDH and ADH.
Trilostane
Trilostane is a competitive inhibitor of 3-beta-hydroxysteroid dehydrogenase.
Trilostane reaches peak concentrations 2 hours after administration with return to baseline at 10 to 18 hours.
Administration with food enhances absorption.
The manufacturer recommends a starting dose of 2.2-6.7 mg/kg PO every 24 hours.
More recent studies have suggested better and faster control of signs with administration every 12 hours, likely because the duration of action is <13 hours in most dogs.
The author uses a starting dose of 0.5-1 mg/kg PO every 12 hours.
Monitoring of dogs treated with trilostane has shifted, with studies showing that ACTH stimulation testing does not correlate well with clinical response.24-27
Monitoring should focus on resolution of clinical signs. A pet owner questionnaire can be used to help track improvement.
Dogs that are clinically well with controlled or uncontrolled signs of HAC can likely be monitored using pre-pill cortisol concentrations.
Dogs that are not clinically well should be assessed with an ACTH stimulation test.
Initial rechecks should be 10 to 14 days and 30 days after therapy is initiated.
Previously, ACTH stimulation testing was recommended at 10 to 14 days. The author no longer includes this unless concern for hypoadrenocorticism is present. Clinical signs should be assessed instead. The dose is not typically altered at the 10 to 14 day visit unless clinical signs of hypoadrenocorticism are present.
At the 30-day recheck, signs of HAC should be assessed and pre-pill cortisol measured.
If signs (eg, PU/PD, polyphagia) are improved and pre-pill cortisol is 1.5-5 micrograms/dL, the current dose should be maintained and the patient rechecked in 3 months.
If signs persist and pre-pill cortisol is >5 micrograms/dL, the dose should be increased by 25% to 50% and the patient rechecked in 4 weeks.
If signs persist and pre-pill cortisol is 1.5-5 micrograms/dL, the dose should either be increased or, if administration has been every 24 hours, split and administered every 12 hours.
If signs persist and pre-pill cortisol is <1.5 micrograms/dL, clinical signs should be reassessed. Consideration can be given to performing an ACTH stimulation test to assess control. Increasing the dose, or splitting the dose with administration every 12 hours (if being given every 24 hours), should be considered.
Mitotane
Mitotane is an adrenocorticolytic agent that predominately targets the zonas fasciculata and reticularis.
Mitotane is uncommonly used except in cases refractory to trilostane therapy.
Treatment includes 2 phases: induction and maintenance.
In the induction phase, the initial dose is 30 to 50 mg/kg PO per day, usually split and administered every 12 hours and continued until signs decrease (eg, slower to eat, decreased PU/PD) or for a maximum of 7 to 10 days.
ACTH stimulation testing should be repeated at that time to assess therapy, with the goal of post-ACTH cortisol concentration within the baseline cortisol reference interval (1-5 micrograms/dL).
In the maintenance phase, the total daily induction dose should be used as the total weekly maintenance dose, typically split and administered over 3 to 4 days per week.
Monitoring should include assessment of signs and ACTH stimulation testing 4 weeks after maintenance therapy is initiated, then as needed if dose adjustments are made or signs return.
The following should be considered when comparing trilostane with mitotane.
Trilostane has similar adverse effects compared with mitotane, but they are typically less severe.
Trilostane may be a better option for patients with owners concerned about significant adverse effects.
Trilostane can be more easily adjusted than mitotane (ie, change in dose versus reinduction) in dogs in which control is lost.
Mitotane is uncommonly used but remains an option for treating dogs nonresponsive to trilostane or in dogs with HAC that become refractory to trilostane therapy.
In some cases, mitotane may be a less expensive option in larger dogs.
Prognosis
Prognosis for dogs with PDH treated medically is good, with a reported median survival of 600 to 900 days with trilostane administration.28-31 In the author’s experience, significant HAC-associated mortality is rare, except in cases of macroadenomas or in which PU/PD cannot be controlled. Dogs with neurologic signs from a macroadenoma have a poorer prognosis but may do well with radiation therapy and medical treatment.32
Prognosis for dogs with ADH is good to excellent with complete excision of adrenal tumors without metastasis. Medical therapy for ADH has a fair to good prognosis, with a reported median survival of 14 to 15 months.33