
Additional background information on persistent or suspected-resistant hookworm infections is available here.
Studies have provided evidence that drug resistance in the canine hookworm, Ancylostoma caninum, is prevalent throughout the United States and an important health concern for dogs.1-3
One study showed that multiple anthelmintic drug resistance (MADR) in canine hookworms may be ubiquitous in racing and recently retired greyhounds1; however, resistance is a concern in all breeds.2 For example, hookworms with MADR were associated with clinical disease in Labrador retrievers in a kennel with no known association with greyhounds.3
Evidence indicates MADR in canine hookworms did not develop recently. Genetic findings demonstrated ≈50% of pet dogs infected with hookworms harbored benzimidazole (eg, fenbendazole, febantel)-resistant hookworms,2 suggesting these parasites have been circulating for a significant length of time. Of note, only resistance to benzimidazoles was investigated in the study because this is the only drug class with genetic markers for resistance. The percentage of infections that included hookworms also resistant to pyrantel and/or macrocyclic lactones is thus unknown, but clinical evidence suggests many benzimidazole-resistant hookworm infections in pet dogs are likely resistant to multiple anthelmintics.4
Diagnosing Drug Resistance in Hookworms
Clinical vigilance is important, as patients diagnosed with hookworm infection may harbor drug-resistant parasites. A positive fecal test result for A caninum could be a result of primary infection, larval leak (ie, larvae arrested in somatic tissue repopulating the intestine and shedding eggs), reinfection, and/or drug resistance. Anthelmintic resistance should be strongly suspected in infected dogs recently administered a drug labeled for treatment of hookworm infections (eg, monthly heartworm/intestinal worm prophylaxis), as the drug should have killed susceptible hookworms; additional diagnostics should be pursued. In contrast, infected dogs not recently administered an anthelmintic are likely infected with genetically diverse hookworms; none, few, or many of these may be resistant.
A fecal egg count reduction test (FECRT) is needed to determine which drugs hookworms are resistant to, as FECRT can test resistance to a single anthelmintic or a combination of anthelmintics, including pyrantel and/or macrocyclic lactones (see Steps for Performing a Fecal Egg Count Reduction Test in a Dog).
Steps for Performing a Fecal Egg Count Reduction Test in a Dog
Step 1
Perform 2 independent fecal egg counts (FECs) on a single fresh (ie, collected <12 hours after defecation, refrigerated prior to testing) fecal sample.
Author Insight
At least 50 eggs should be counted under the microscope. If FECs are high (>600 eggs per gram [EPG]), eggs can be counted using the McMaster method.5 If EPG is low, another FEC should be conducted or an FEC method with a lower multiplication factor (eg, Mini-FLOTAC) used.6 If conducting FECRT in a kennel, 6 dogs should be included in the treatment group, and a total of at least 300 eggs should be counted among the 6 dogs.
Step 2
Administer the drug given previously (for which resistance is suspected), following the label instructions.
Step 3
Collect a posttreatment fecal sample on day 14 (10-14 days is acceptable), and perform 2 FECs.
Author Insight
The same number of FECs and method must be performed on pre- and posttreatment samples.
Step 4
Calculate and interpret the percentage reduction in FEC using the following equation:
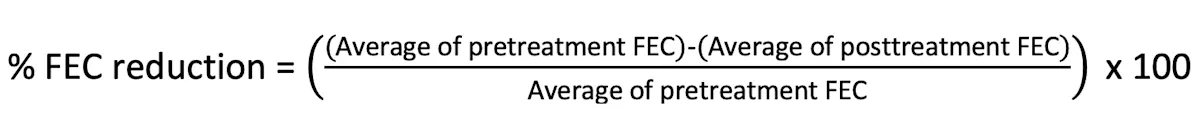
Author Insight
If resistance is confirmed or strongly suspected, a triple combination (ie, macrocyclic lactone, benzimidazole, tetrahydropyrimidine; see Treatment & Testing Strategy) should be administered and FECRT repeated. Management should be based on results of the second FECRT.
Fecal Egg Counts
To perform FECRT, the eggs per gram (EPG) of feces must be quantified before and after treatment using a fecal egg count (FEC). Fecal flotations are frequently performed in small animal practice but are inadequate for FECRT, as they provide qualitative, not quantitative, results; for example, fecal flotation results are often categorized as rare, few, moderate, or many. A study performed in the author’s laboratory (unpublished data, 2021) showed a high level of FEC variation within, and overlap among, these categories. The preferred quantitative FEC methods are McMaster and Mini-FLOTAC.5,6 New semiautomated fecal analysis systems use artificial intelligence and machine learning and may eventually replace older methods; however, few systems capable of performing FEC have been developed, and none are validated for FECRT use in dogs.
All FEC methods involve dilution; therefore, a multiplication factor is applied to the number of eggs counted under the microscope to calculate EPG. Multiplication factors vary (eg, Mini-FLOTAC, ×5 EPG; McMaster, ×25 or ×50 EPG [depending on protocol]); smaller multiplication factors lead to more eggs counted under the microscope. Because the number of eggs counted in the pretreatment FEC is key when performing FECRT, the sample’s EPG influences selection between the McMaster and Mini-FLOTAC methods. Although EPG is not known until FEC is complete, a centrifugal flotation can be performed first; if many eggs are seen, the McMaster method can be selected, and if few or moderate numbers are seen, the Mini-FLOTAC method should be used.
Posttreatment Fecal Egg Count Timing
Performing FEC too soon after treatment with a benzimidazole can yield a false-negative result for resistance. In contrast, if too much time passes, larval leak can lead to a false-positive interpretation. Few data exist on time to worm maturity and egg production in adult dogs with chronic infections; however, a clinical trial conducted by the author in greyhounds infected with hookworms with MADR (unpublished data, 2020) indicated hookworms leaking from somatic tissues can repopulate the small intestine and begin shedding in 3 to 5 weeks. A posttreatment fecal sample should be collected 10 to 14 days (optimal, 14 days) after treatment is completed.
Treatment & Testing Strategy
If resistance to an anthelmintic is diagnosed (Table 1), testing individual drugs for efficacy is impractical. The patient should be retreated with a macrocyclic lactone, a benzimidazole, and a tetrahydropyrimidine (ie, triple combination) to maximize efficacy. Repeating FECRT after administration of the triple combination is crucial to determine whether treatment was successful.
Topical moxidectin is the anthelmintic most likely to remain efficacious; however, MADR, including resistance to moxidectin, was highly prevalent in greyhounds in a recent study.1 All products containing moxidectin are not equal. The topical moxidectin product uses a higher dose (2.5 mg/kg) than other canine products containing moxidectin. Although the efficacy of other products containing moxidectin has not been similarly evaluated against hookworms with MADR, the large dose differential suggests those products are unlikely as effective as the topical product.
Interpretation of Fecal Egg Count Reduction Test Results
In the absence of drug resistance, anthelmintic treatment is typically highly effective, with few or no eggs seen in posttreatment fecal tests; however, factors unrelated to resistant hookworms can cause reduced efficacy. A reliable diagnosis of resistance can only be established if the patient was administered the proper drug dosage, the drug was within the expiration date and stored properly, fecal samples were labeled and stored correctly prior to analysis, FECRT was performed properly with a correctly timed posttreatment FEC, proper laboratory techniques were applied when FEC was performed, the same FEC method was used on both pre- and posttreatment samples, and a sufficient number of eggs (at least 20, preferably at least 50) were counted in the pretreatment FEC.
Guidelines for interpreting FECRT results differ depending on whether a single drug or multiple drugs were administered (Tables 1 and 2).
Table 1: Interpretation of Fecal Egg Count Reduction Test Results Following Administration of One Anthelmintica,b
a At least 50 eggs should be counted in pretreatment FECs. Errors in interpretation are more likely if only 20 to 50 eggs are counted. Accurate interpretation is difficult if <20 eggs are counted.
b Drugs approved for treatment of hookworms include fenbendazole, febantel, pyrantel, milbemycin oxime, moxidectin, and selamectin. Interpretations in this table are based on administration of only one of these drugs.
Table 2: Interpretation of Fecal Egg Count Reduction Test Results Following Administration of a Triple Combinationa,b
a At least 50 eggs should be counted in pretreatment FECs. Errors in interpretation are more likely if only 20 to 50 eggs are counted. Accurate interpretation is difficult if <20 eggs are counted.
b Triple combination comprises a macrocyclic lactone, a benzimidazole, and a tetrahydropyrimidine.
When a triple combination is administered, thresholds for FEC reduction are stringent because each anthelmintic should have an additive effect. For example, if each drug has 80% efficacy, the first drug will provide 80% efficacy, the second drug will provide 80% of the remaining 20% (16%), and the third drug will provide 80% of the remaining 4% (3.2%), yielding an efficacy of 99.2%. Even if all 3 drugs have reduced individual efficacy, high efficacy should be easily achieved. Because of this likelihood for efficacy, administration of a triple combination is recommended when resistance to an anthelmintic is identified and interpretation of results differs from the use of single drugs.
Avoiding Overinterpretation
FECRT is fairly sensitive for resistance detection (because dead worms do not shed eggs). Once a reduction in efficacy is observed, however, the measured reduction in percentage does not provide much additional information because several factors (eg, variation in level of egg shedding among worms, increase in egg production in resistant worms following treatment that kills some worms [ie, density-dependent fecundity]) can cause variability in FEC and influence the FEC reduction percentage measured in a single test.7 The impact of these factors is magnified when only a single dog is tested.
Emodepside
Emodepside is the only potentially effective alternative treatment in cases in which a triple combination is shown to be ineffective via FECRT. Dogs infected with heartworms should not be treated with emodepside due to potential adverse drug effects.
Follow-Up
In patients with persistent egg shedding, FEC (not just flotation) should be evaluated at regular intervals. The optimal interval varies based on the patient and level of pet owner concern; however, performing FEC every 3 to 4 months is likely reasonable. Yearly fecal examinations are inadequate, as infections with resistant hookworms should be diagnosed in a timely manner to prevent infection levels from increasing, possibly leading to clinical disease and spread of infective larvae to other dogs.
Reinfection with resistant hookworms increases resistance, further reducing treatment efficacy. This is especially important when treating hookworms resistant to avermectin/milbemycin drugs (ie, selamectin, milbemycin) with moxidectin. Reinfection with offspring of avermectin/milbemycin-resistant hookworms that survived moxidectin treatment can lead to rapid development of moxidectin resistance.8 In addition, hookworm eggs shed in feces following administration of a triple combination are highly resistant to all anthelmintics. Reinfection can occur if these eggs develop to infective third-stage larvae, increasing the level and spectrum of resistance and facilitating further spread of hookworms with MADR.
Recommendations in this article are based on the author’s interpretation of best available evidence at the time of publication and are likely to change based on future research.
Listen to the Podcast
Want to learn more? Listen to Dr. Kaplan share more of his expert insights.