Honey in Wound Management
Crystal R. Zimmerman, CVT, LVMT, University of Tennessee
Karen M. Tobias, DVM, MS, DACVS, University of Tennessee
Honey has historically been used to treat difficult wounds, and interest in its therapeutic properties has increased with the emergence of drug-resistant bacteria.1-6
This clinical overview compares uses of table honey and medical honey as a wound dressing, offers notes on wound-healing effects of honey, and provides a brief explanation on the composition of honey. Image-based examples from 3 cases of wound management are included, as well as a quick-reference chart for honey dressing based on wound characterization.
Medical Effects of Honey
As a medicant, honey is most often used for wound management, particularly because of its antibacterial activity, which results from high osmolarity, low free-water content, acidity, hydrogen peroxide formation with dilution, and nonperoxide antimicrobial substances (eg, methylglyoxal, bee defensin-1; Production & Composition).2,5,7,8 Both table and medical-grade honey can be effective in managing wounds (Potential Wound-Healing Effects); benefits of medical-grade honey include greater consistency in composition, quality, sterility, and bactericidal activity.1
Table Honey
Table honey commonly contains nonpathogenic microbes, but small numbers of Enterobacteriaceae (Proteus spp) and other pathogenic organisms are occasionally cultured1,8-10; however, there have been no clinically documented cases of wound infection associated with table honey.
Table honey can eliminate microbes in wounds, decrease wound area, and promote re-epithelialization.11 In one study of burn wounds in humans, daily dressings with table honey resulted in elimination of bacteria in wounds within 7 days and complete healing within an average of 18 days.12 In comparison, patients who received silver sulfadiazine dressings had positive cultures at 7 days, and average time to heal was 32.7 days.
Medical Honey
Heating honey can reduce antimicrobial effectiveness; medical-grade honey is thus usually sterilized via gamma irradiation.13-16 Two common types of medical honey are honey from the manuka (Leptospermum) tree and greenhouse-produced hydrophilic honey gel.17
Honey from the Manuka Tree
Many antimicrobial effects of manuka honey come from methylglyoxal, a phytochemical thought to inhibit cell division of gram-positive bacteria and alter gene expression of structural proteins in gram-negative bacteria.17 The amount of expected antibacterial activity in medical manuka honey is identified by its unique manuka factor (rating of 16-30 indicates high methylglyoxal content).8,17
Greenhouse-Produced Hydrophilic Honey Gel
Antimicrobial activity of greenhouse-produced honey is a result of hydrogen peroxide formation, which occurs from the action of glucose oxidase on glucose, oxygen, and water. Peroxide content, and thus antimicrobial activity, increases in the dressing as the greenhouse-produced honey is diluted by wound exudate.18 This honey also contains bee defensin-1, an antimicrobial and immunomodulatory peptide.
Manuka and greenhouse-produced honey are bactericidal against methicillin-resistant Staphylococcus spp and other various bacterial species after 24 hours of incubation, even when diluted to 5 to 60 times their volume (Figure 1).18,19 Unlike antibiotics, acquired resistance in wounds has not been documented with topical honey.10
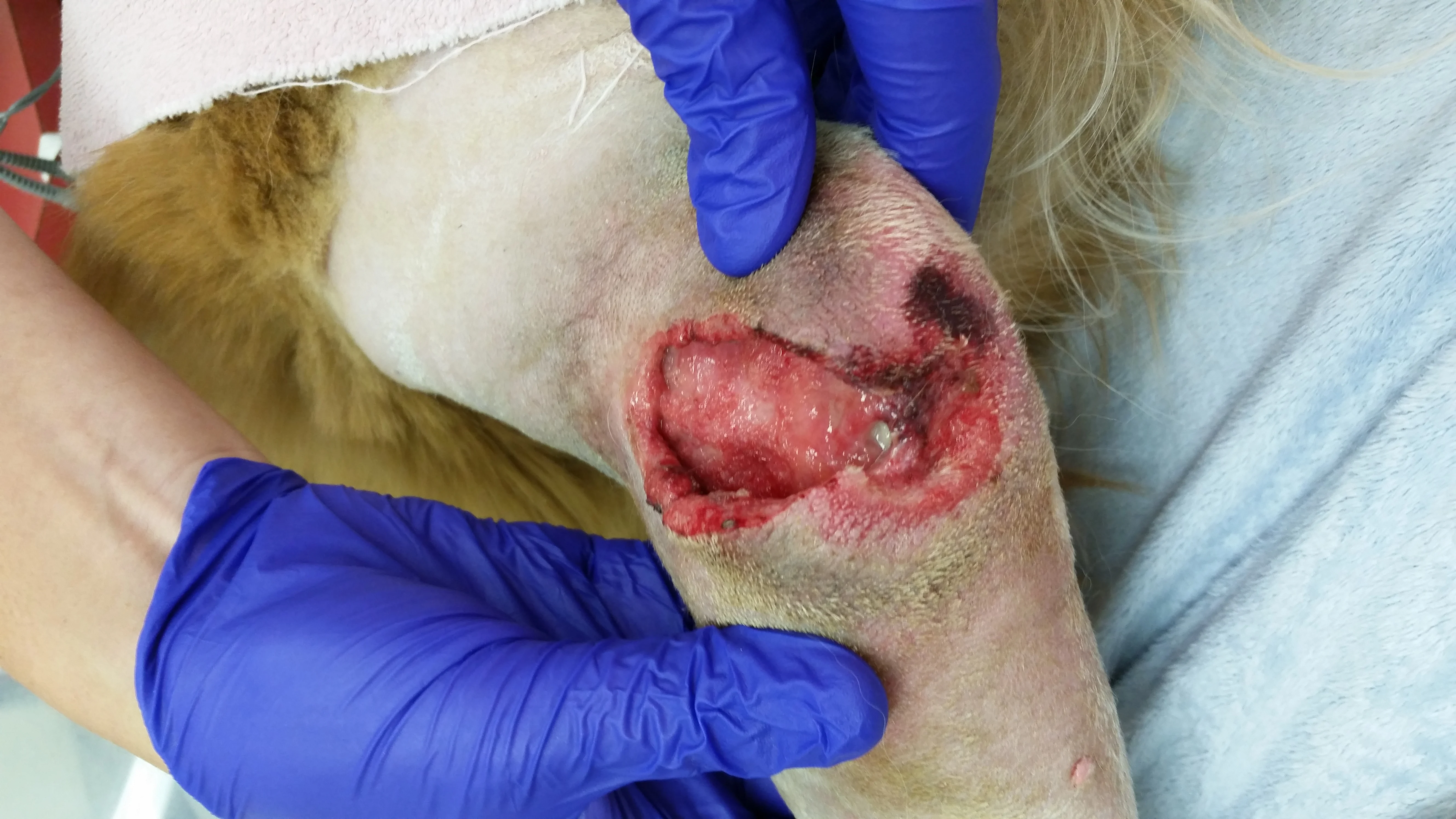
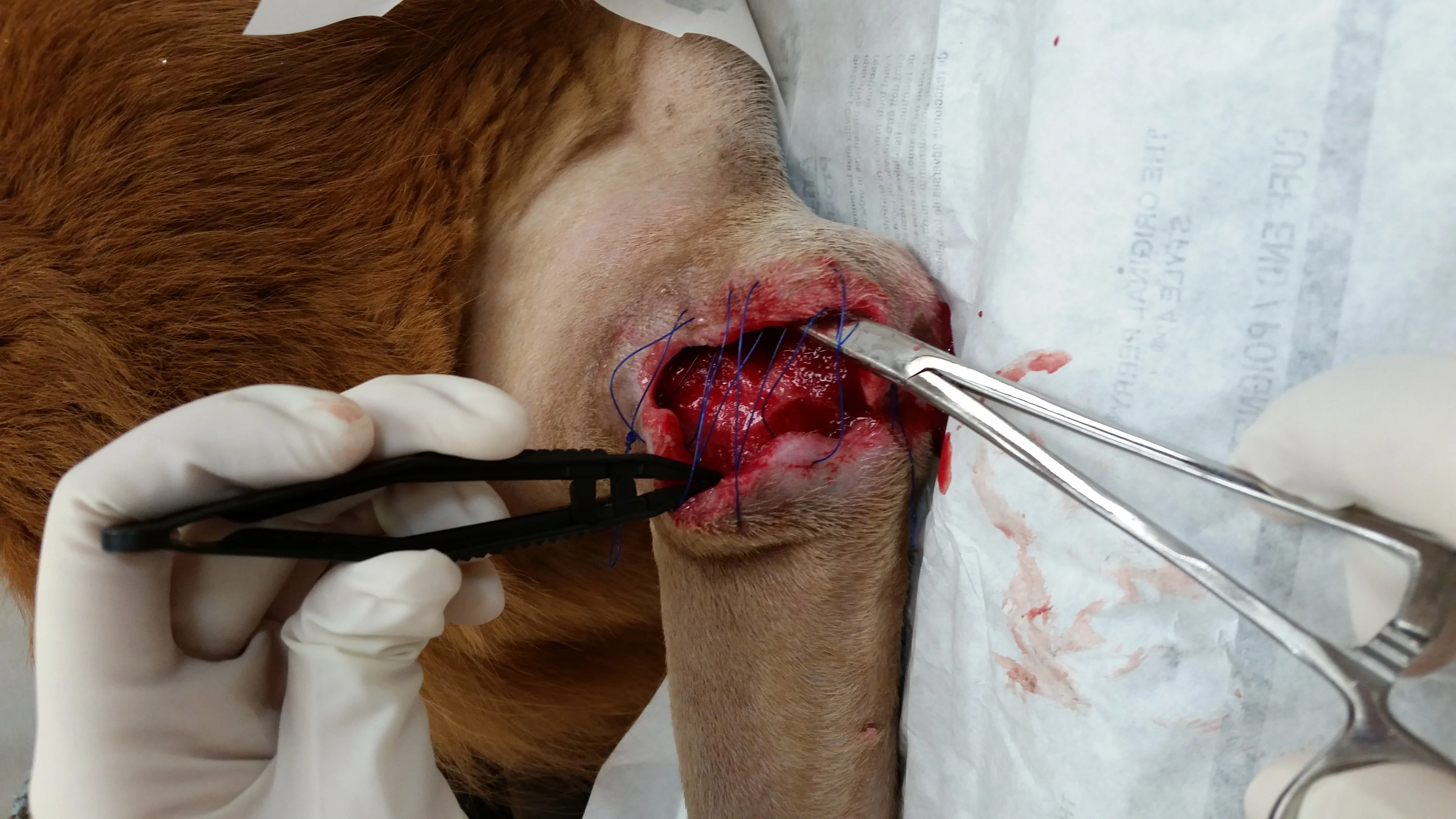
Surgical dehiscence after elbow hygroma resection (A). Topical honey was used in the wound bed to treat methicillin-resistant Staphylococcus spp and encourage granulation tissue formation. After 4 days of treatment, the wound is filled with healthy granulation tissue and ready to close (B).
Clinical Use of Honey
Dressing selection depends on the stage of the wound, amount of fluid produced by the wound, and desired effect in the wound. Medical-grade honey dressings include pastes, gels, hydrogel colloidal sheets, and calcium alginate sheets (Honey Dressing Based on Wound Character).20,21 Honey paste displays similar consistency to refrigerated honey, and once warmed, the paste liquefies and flows into fissures, cavities, and other areas that are hard to reach. Liquid honey gel contains a natural gelling agent that makes it more stable and thus more likely to remain in place. Honey-impregnated calcium alginate sheets dissolve as they absorb wound fluid, simultaneously releasing honey and forming a gel that locks fluid in place. Honey-impregnated hydrogel colloidal sheets absorb a small amount of fluid but primarily maintain a moist environment in wounds with minimal drainage.
Daily bandage changes and absorptive dressings may be necessary because honey encourages exudate formation. In dogs that will eventually undergo surgical closure of wounds (rather than second intention healing), honey dressings can be applied until the wound is filled with a healthy granulation bed and surrounded by a fresh rim of epithelium (usually indicating control of local infection), at which point the wound can be closed safely.
Once wounds start to granulate, treatment goals are to maintain a moist environment, provide a local antimicrobial effect, encourage granulation tissue formation, and protect delicate tissues as they form and migrate. A gel or hydrogel colloidal sheet honey dressing can be effective when paired with an appropriate secondary layer. Epithelializing wounds are usually not infected, and the primary goal of topical dressing should be to keep the area protected and slightly moist to allow keratinocyte migration; these wounds usually do not need honey.
Case 1: Mildly Exudative, Likely Infected Bite Wound
A patient is presented with a deeply pocketed bite wound that has a small amount of thick exudate. The wound is first filled with water-soluble lubricant and clipped widely (Figure 2). Loose hairs and lubricant are rinsed out of the wound and surrounding area, which are then scrubbed with 4% chlorhexidine and rinsed with sterile saline or tap water. Necrotic tissue is debrided with sharp dissection or gauze abrasion (Figure 3). A tissue sample of the wound bed is obtained for culture; then wide-meshed, antimicrobial gauze impregnated with honey paste is inserted into the pockets (Figure 4). The wound is covered with an absorptive pad secured with a tie-over bandage, which should be changed within 24 hours, as honey paste increases the amount of fluid production.
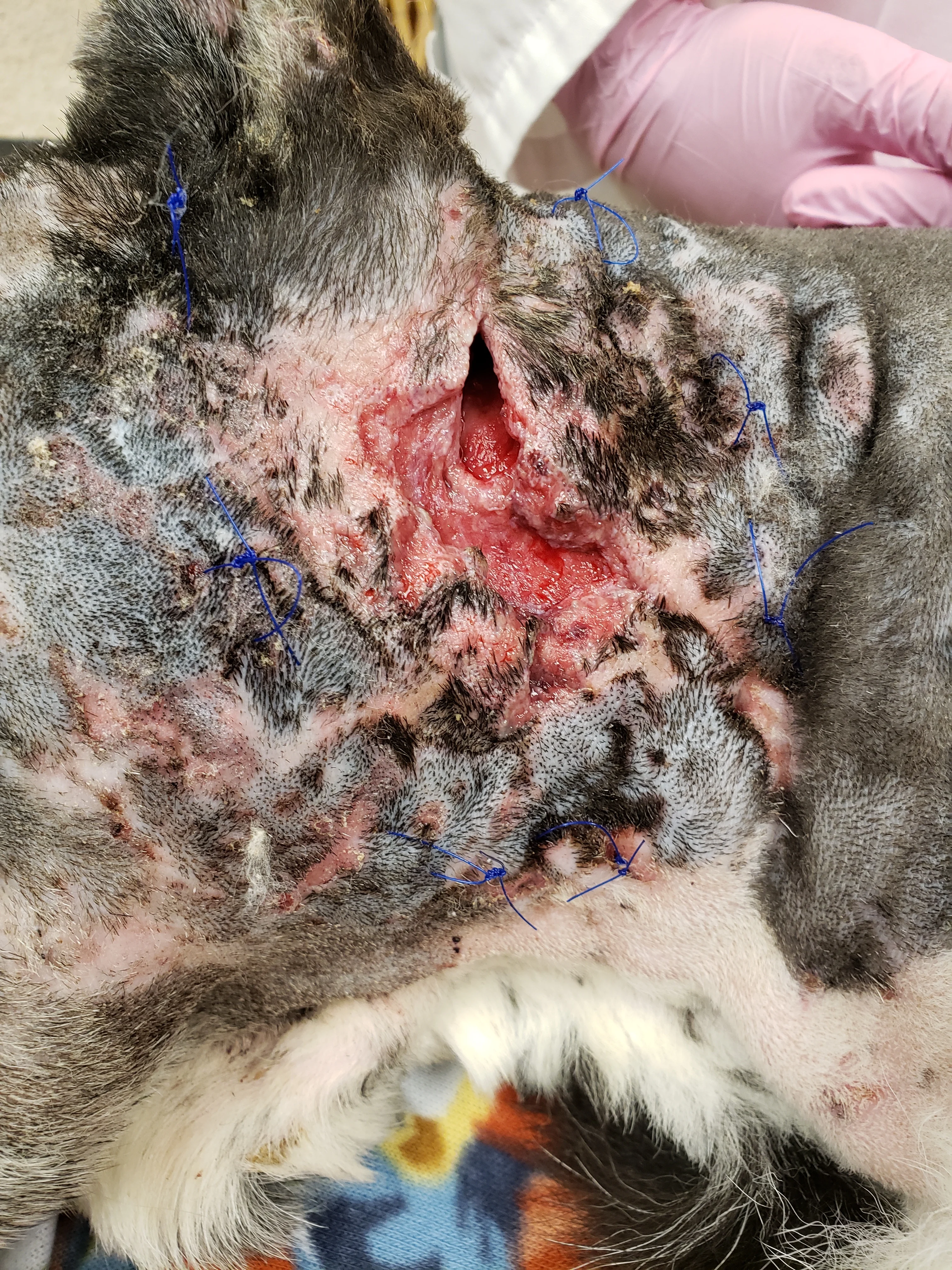
Figure 2
Neck bite wounds after initial debridement and placement of suture loops for tie-over bandage
Case 2: Traumatic, Moderately Effusive Inguinal Wound
A patient is presented with a moderately effusive inguinal wound caused by vehicular trauma. The wound is first clipped, cleaned, and flushed (Figure 5). Deep tissue is obtained for culture, and sutures are placed for a tensioning or tie-over bandage (Figure 6). Honey calcium alginate pads (Figure 7) are placed in the wound (Figure 8). After 24 hours, the pads begin to have a gel consistency as wound fluid is absorbed (Figure 9); local dermatitis has improved, and granulation tissue is beginning to fill the wound (Figure 10). Infection is likely still present, and continued open management or debridement is needed before closure. The wound is temporarily stapled closed for tibial fracture repair (Figure 11).
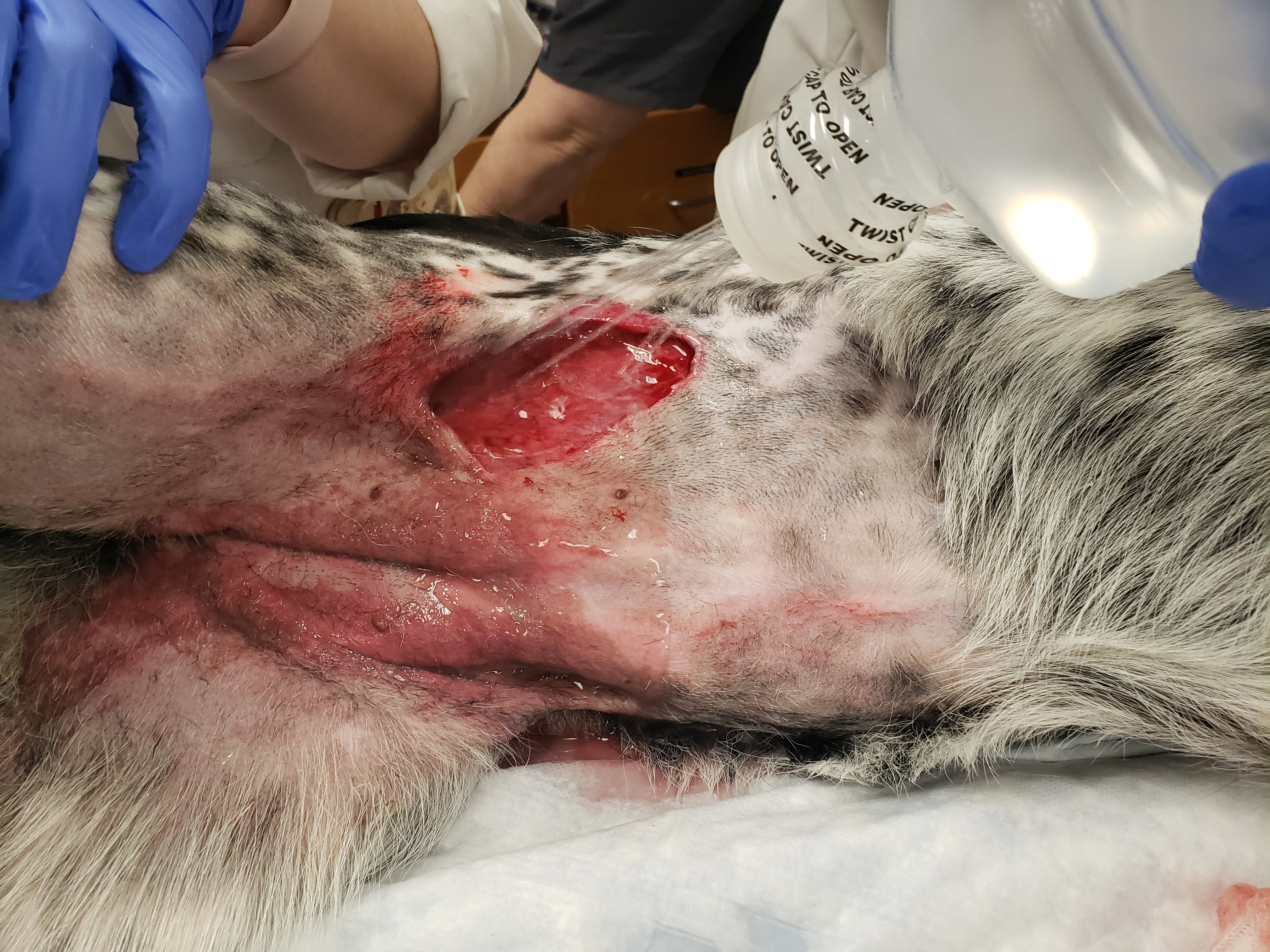
Figure 5
Open wound from vehicular trauma is clipped widely and prepped. Warm sterile saline is sprayed through cap holes made by an 18-gauge needle. Extensive dermatitis from wound fluid drainage can be seen.
Case 3: Pavement Burns
Patient is presented for pavement burns after being dragged by a car (Figure 12). Extensive wounds on the body include partial- and full-thickness pavement burns, swelling, and open wounds (Figure 13). Wounds are clipped, cleaned, and treated with surgical debridement and honey. Honey gel is applied directly on eschars, sloughing or necrotic tissues, and dry exposed bone; gauze and a super-absorbent pad are applied to wounds with thin effusion; and honey paste/gel and absorptive gauze/foam are applied for limb degloving. A tensioning suture is used to hold the dressings in place. After 2 days of topical honey administration, granulation tissue is present (Figure 14). After 3 days, there is a rim of new epithelium on the thoracic wound, likely indicating infection control (Figure 15). Partial-thickness eschars (eg, on the right hip) are treated with honey gel, and full-thickness eschars are surgically resected. Honey-impregnated gauze is held in place with tensioning sutures that are gently tightened and secured with fishing sinkers (Figure 16). Stapled and encircling bandages are also used in some areas. Petrolatum-impregnated, knitted cellulose acetate, nonadherent dressing is stapled over foot wounds to provide pain relief and protect the tissue while allowing fluid efflux and topical application of antimicrobials (Figure 17).
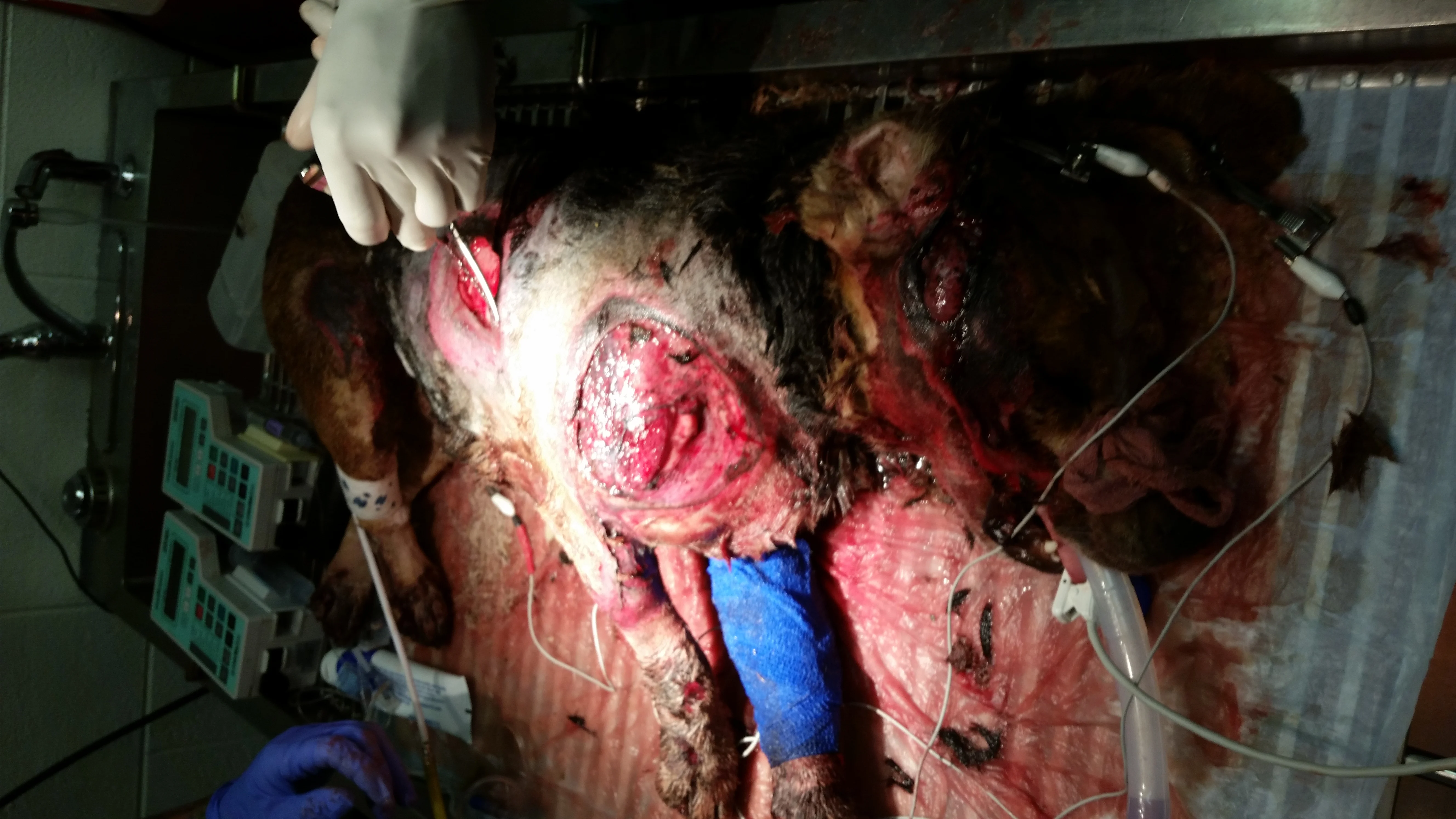
Figure 12A
Extensive pavement burns after inadvertent dragging by a car. The dog is intubated (head is to the right and feet are toward the bottom of the image).
Conclusion
Topical honey used appropriately can be an effective wound dressing.2 Honey can decrease microbial counts, reduce inflammation, keep wounds moist, facilitate debridement, modulate the local immune reaction, and stimulate wound healing.17,22-25 Medical-grade honey is more costly but provides customized formulations, sterility, and consistency.
Production & Composition
Honeybees produce and store honey as a food source for the hive.26 Foraging bees collect nectar from flowers and temporarily store it in their crops (ie, proventriculus, honey sac), where salivary enzymes and proteins help break down sugars. Bees then return to the hive, regurgitate the nectar, and transfer it to house bees that repeatedly ingest and regurgitate the nectar until sugars, starches, and proteins are partially or completely digested and some moisture has evaporated, increasing the acidity of the honey. Bees place the nectar in open honeycombs and fan the surface to maintain air circulation and increase the temperature of the hive, further reducing the water content of the honey so it can be safely sealed in the combs without risk for fermentation.26
Honey composition—including sugars (primarily fructose and glucose), water (usually ≈17%), enzymes (eg, glucose oxidase), other proteins (eg, bee defensin-1), vitamins, minerals, amino acids, antioxidants, and phenolic acids—varies with plant origin, season of collection, geographic location, health of foraging bees, exposure of harvested honey to heat or light, and storage time.2,5,7,13,27,28
Potential Wound-Healing Effects
Stimulation of fibroblast and macrophage activity secondary to low pH
Stimulation of vascular endothelial growth factor production by peroxide
Reduction in bacterial proteases that destroy cytokines, growth factors, and extracellular matrixes17,22,23,29
Disruption or prevention of biofilm formation17,23
Immunomodulatory effects (eg, activation of receptors that enhance production of cytokines important in tissue repair and regeneration)17,22
Reduction in inflammation via decreased activity of cyclooxygenase-1 and -2 and decreased production of inflammatory prostaglandins and thromboxane B2, a potent vasoconstrictor24
Autolytic debridement23,25
Viscosity creates a surface layer that inhibits bacterial entry while protecting the wound from dehydration.
Drawing water out of tissue encourages influx of oxygen and nutrients.23