Gastric Dilatation-Volvulus in Dogs
Melisa Thompson, DVM, Tuskegee University
W. Alex Fox-Alvarez, DVM, MS, DACVS-SA, Community Care Veterinary Specialists, Gainesville, Florida

Gastric dilatation-volvulus (GDV) most commonly affects large- to giant-breed dogs and is a surgical emergency characterized by rapid gastric distention and 270-degree to 360-degree rotation of the stomach on the mesenteric axis.1,2 Massive gastric distention prevents normal respiratory excursions and compresses vital abdominal vasculature, leading to hypovolemic shock, arrhythmia, electrolyte disturbance, visceral necrosis, organ failure, sepsis, and death.1-3 Mortality rates range from 10% to 33% despite treatment.4 Early surgical intervention is recommended; however, efficient preoperative patient stabilization is crucial.
Pathophysiology
The underlying cause of GDV remains unknown.5 GDV appears to be multifactorial and may vary among patients, breeds, and scenarios. Risk factors can be categorized as intrinsic (eg, genetics, conformation, personality, age) or extrinsic (eg, diet, activity, environment). Dogs with family history of GDV,6 large and giant purebred dogs, and dogs with a higher thoracic depth:width ratio are reportedly at higher risk.1,2 Nervous, fearful, and aggressive dogs are at greater risk than calm dogs.6,7 Risk also increases with age; however, older dogs do not have a higher mortality rate with treatment and should still be considered appropriate surgical candidates.8 Large meal volume, once-daily feeding, rapid eating habits, and diets with oils or fats in the first 4 listed ingredients are additional risk factors in breeds that already have increased risk.9
Literature evaluating splenectomy as a risk factor has yielded conflicting results.10,11 One study found that previous splenectomy patients were 5.3 times more likely to develop GDV.10 In addition, uncommon breeds (eg, beagles, dachshunds, bichons frises) have been reported to develop GDV after splenectomy.11 The authors routinely perform gastropexy at the time of splenectomy in dogs with additional risk factors for GDV.
History & Clinical Signs
Typical presentation includes large, deep-chested dogs with recent history of abdominal distention, panting, restlessness, hypersalivation, and repeated nonproductive retching. Clinical signs vary based on degree of rotation and duration of GDV. Some dogs are bright and alert, whereas others may be recumbent or comatose.12 Patients may be presented in varying degrees of shock.12 Common clinical signs include tachycardia, poor peripheral pulses, capillary refill time <1 second, decreased temperature, and gastric tympany.13
Diagnosis
Presumptive diagnosis can be made based on signalment, history, and physical examination findings. Abdominal radiography can help confirm diagnosis and exclude gastric dilatation alone or food bloat.2,14,15 Right lateral, left lateral, and dorsoventral positions are suitable for diagnosis via radiographs. Dorsoventral positioning is preferred over ventrodorsal to minimize stress and decrease the risk for aspiration pneumonia from inversion.
The classic double bubble appearance of GDV in the right-lateral view represents a gas-distended gastric lumen with the pylorus located craniodorsal to the fundus. Pylorus and fundus are divided by a soft tissue opaque band that compartmentalizes the gas in the fundus and pylorus, making 2 bubble shapes (Figure 1). Focal gas opacities in the gastric walls may suggest necrosis, and free gas in the abdomen can indicate gastric perforation.15
Early in the course of disease, CBC results may only reveal a stress leukogram. Hemoconcentration, thrombocytopenia, and leukopenia can occur as GDV progresses, indicating onset of septicemia and disseminated intravascular coagulation.2,15,16 Prerenal azotemia, elevations in liver enzymes due to tissue hypoxia, and mild to moderate electrolyte disturbances may be noted. GDV causes mixed acid–base disturbances, and pH can be elevated, decreased, or normal.2,15,16
Several studies demonstrate a relationship between initial plasma lactate values and morbidity/mortality17-22; however, there is considerable variability and overlap between survivors and nonsurvivors, making initial lactate a poor prognostic indicator (Table). The response of lactate to stabilization efforts should instead be used to help guide resuscitation and prognostication. A decline in plasma lactate of >40% to 50% after fluid resuscitation suggests a significantly greater chance of survival, regardless of the initial value.4,17
Electrocardiography should be performed to evaluate for ventricular arrhythmias secondary to myocardial ischemia, electrolyte disturbances, and reperfusion injury.14
Lactate Cutoffs Used To Predict Mortality*
*Variability and overlap in values and mortality rates can be noted.
Treatment
Stabilization
Aggressive fluid resuscitation and gastric decompression are necessary for optimal anesthesia.14 A large-bore IV catheter should be placed in one or both cephalic veins, as venous return from the caudal half of the body is compromised. An opioid should be administered to relieve discomfort, and a balanced crystalloid (shock dose, 90 mL/kg in quarter volume increments until vital parameters improve) should be provided rapidly. If the patient remains hypotensive, vasopressor therapy may be administered.14,23
Gastric decompression is critical to allow restoration of normal perfusion. Decompression is performed via orogastric intubation or trocarisation, while shock fluid therapy is ongoing. Orogastric intubation is performed with the patient under sedation and with a lubricated polyvinyl chloride (ie, PVC) tube measured from the tip of the nose to the xiphoid process. Gastric lavage using a drench pump may be helpful for thicker material. The tube should be kinked during removal to prevent material from exiting the tube and entering the airway as the tube is removed. Gastric lavage in a sedated patient without a protected airway may increase risk for aspiration pneumonia, especially when using a drench pump. Endotracheal intubation with a cuffed endotracheal tube can further protect the airway from aspiration during orogastric decompression.
Gastric trocarisation is performed using up to a 14-gauge IV catheter placed percutaneously in the area where tympany is most palpable, typically in the right lateral abdomen, after sterile preparation.15,16 The catheter should be held in place until the tympany resolves and vital parameters improve. Trocarisation of nontarget tissue is possible but not reported to cause clinically significant trauma and can be avoided via ultrasonography of the intended trocar site.24
Trocarisation and orogastric intubation achieve successful gastric decompression in 86% and 76% of cases, respectively.24 The authors prefer trocarisation during resuscitation efforts and orogastric intubation during surgery.
Surgery
Once stabilized, the patient can be anesthetized and prepared for surgery. Perioperative antibiotics (eg, cefazolin; see Suggested Reading) should be administered 30 minutes prior to surgery, and every 90 minutes during surgery. Unless evidence of infection or septicemia are present, antibiotics do not need to be continued postoperatively.
A ventral midline approach to the abdomen can be made from the xiphoid to the caudal third of the abdomen. On entry, if the omentum is draped over the stomach, volvulus is confirmed (Figure 2). The clinician should stand on the right side of the patient and reposition the stomach. The pylorus can be grasped with one hand, pulled ventrally (up), and returned to its usual right-sided location. The opposite hand can push the distended fundus dorsal (down) and to the left of the patient. In complex cases, the passage of an orogastric tube to further evacuate the stomach can help facilitate derotation.
After gastric repositioning, the remainder of the exploratory laparotomy can be completed while gastric perfusion improves. The stomach and spleen can then be evaluated for evidence of necrosis.
Gastric necrosis appears as black or white tissue, is thin on palpation, does not have peristalsis, and should be resected (Figure 3). Necrotic gastric tissue can be resected using GI or transverse anastomotic staplers or with Metzenbaum scissors and a handsewn closure. Placement of an orogastric tube prior to gastric closure can prevent accidental over-narrowing of the lower esophagus and can ensure luminal preservation. Stay sutures can be placed in the healthy gastric tissue surrounding the area of necrosis to minimize spillage during resection and improve tissue control during closure.
Gastric involution can be considered as a quicker alternative in unstable patients, but there is a high rate of gastric ulceration following this technique.25 The necrotic area of the stomach should be oversewn with an inverting pattern (eg, Cushing, Connell) in which all needle bites are taken in healthy gastric tissue adjacent to the necrotic area. This closure brings healthy tissues together and isolates necrotic tissue to the gastric lumen, where it sloughs and passes through the intestinal tract. The splenic hilus should be palpated for pulses, and the parenchyma should be evaluated for color and texture. Splenectomy can be pursued if pulses are absent or color/texture indicates lack of perfusion.14-16
Gastropexy creates a permanent adhesion between the antrum and body wall and dramatically reduces GDV recurrence (Figure 4). Several gastropexy techniques have been described and are similarly effective at preventing recurrence.26 Incisional gastropexy is a simple technique that can be performed open, laparoscopic-assisted, or laparoscopically. Prior to closure, the authors routinely place a nasogastric tube—confirmed via direct gastric palpation—to provide postoperative decompression and an avenue for early feeding.
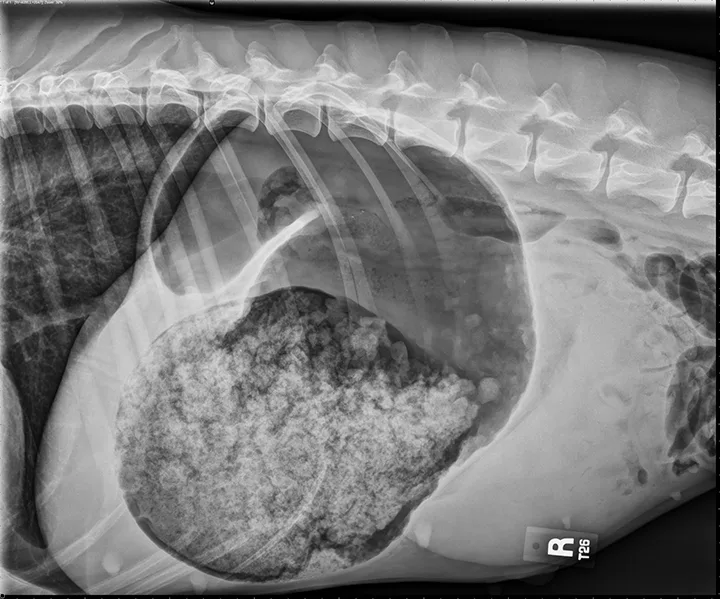
FIGURE 1
The classic double bubble appearance of GDV in the right-lateral view. Distended fundus separated from the gas-distended pylorus by a soft tissue opaque band can be noted.
Postoperative Management
Postoperative hospitalization is needed for supportive care and monitoring, and fluid therapy should be aimed at replacing and maintaining circulating volume. Serial blood gas, electrolyte, glucose, and lactate value analyses can guide fluid therapy and should be checked twice daily in critical patients. Opioids should be administered for analgesia. NSAIDs should not be used due to their negative effects on gastric healing and to avoid renal injury after the relative renal hypoperfusion experienced during GDV. Continuous electrocardiography should be used to monitor arrhythmia so treatment can be given as necessary. Blood pressure should be evaluated postoperatively until normal; some patients may require vasopressors. Antibiotics are not required postoperatively unless there is a secondary need (eg, concurrent aspiration pneumonia, septicemia).
Patients that do not improve or decline after initial improvement may require further diagnostics (eg, coagulation panels, thoracic radiography) to evaluate for the development of disseminated intravascular coagulation, pulmonary emboli, or aspiration pneumonia. Chest radiography should be considered postoperatively in all GDV patients with persistent hyperthermia, as aspiration pneumonia is common.15,16,27
Prognosis & Prevention
The postoperative mortality rate is 10% to 33%.2,14 Factors associated with increased mortality include clinical signs lasting >6 hours,28 presence of gastric necrosis, need for splenectomy,29 and preoperative arrhythmias.30
Without gastropexy, recurrence of GDV can be as high as 80%; effective gastropexy decreases recurrence to <5%.26 Prophylactic gastropexy is recommended in at-risk dogs to reduce risk for GDV and should be performed during routine abdominal procedures in susceptible breeds (eg, Great Danes, German shepherd dogs, standard poodles).2,14,27