Fine-Needle Aspiration
Laura Garrett, DVM, DACVIM (Oncology), University of Illinois
Cytologic examination in small animals has rapidly gained recognition and clinical use. Fine-needle aspiration (FNA) is used to obtain a sample from a mass or organ that will be cytologically evaluated. This powerful tool can be used successfully for a wide variety of anatomic locations and disease processes. In many cases, it can provide a definitive diagnosis, including diagnosis of infections and some tumors.
Even if a definitive diagnosis is not possible, sample evaluation often will rule out many differential diagnoses and point toward the next best diagnostic test. The first step, and an important one, in cytologic analysis is obtaining and preparing a good sample.
Techniques for Sample Acquisition
FNA is the term applied in general to sampling a structure via a needle and subsequently dispelling the needle’s contents onto a slide for cytologic assessment. The term aspiration is often a misnomer because the easiest FNA technique, one that will provide a good sample most of the time, does not actually involve aspiration.
Note that if a mass on an internal organ or an internal lesion can be felt, it can be aspirated. However, for lesions that cannot be palpated, or for lesions near critical structures, such as major blood vessels, ultrasonography or computed tomography (CT) can be useful for guiding FNA (Figure 1).
Unless a culture will be obtained from the aspirate, preparation of the skin before FNA is almost never needed.

CT-guided aspirate of a region of bony lysis within a dorsal spinous process; cytology revealed a sarcoma
What You Will Need
22- or 20-gauge needle
6-mL syringe
5 to 8 glass microscope slides laid out individually
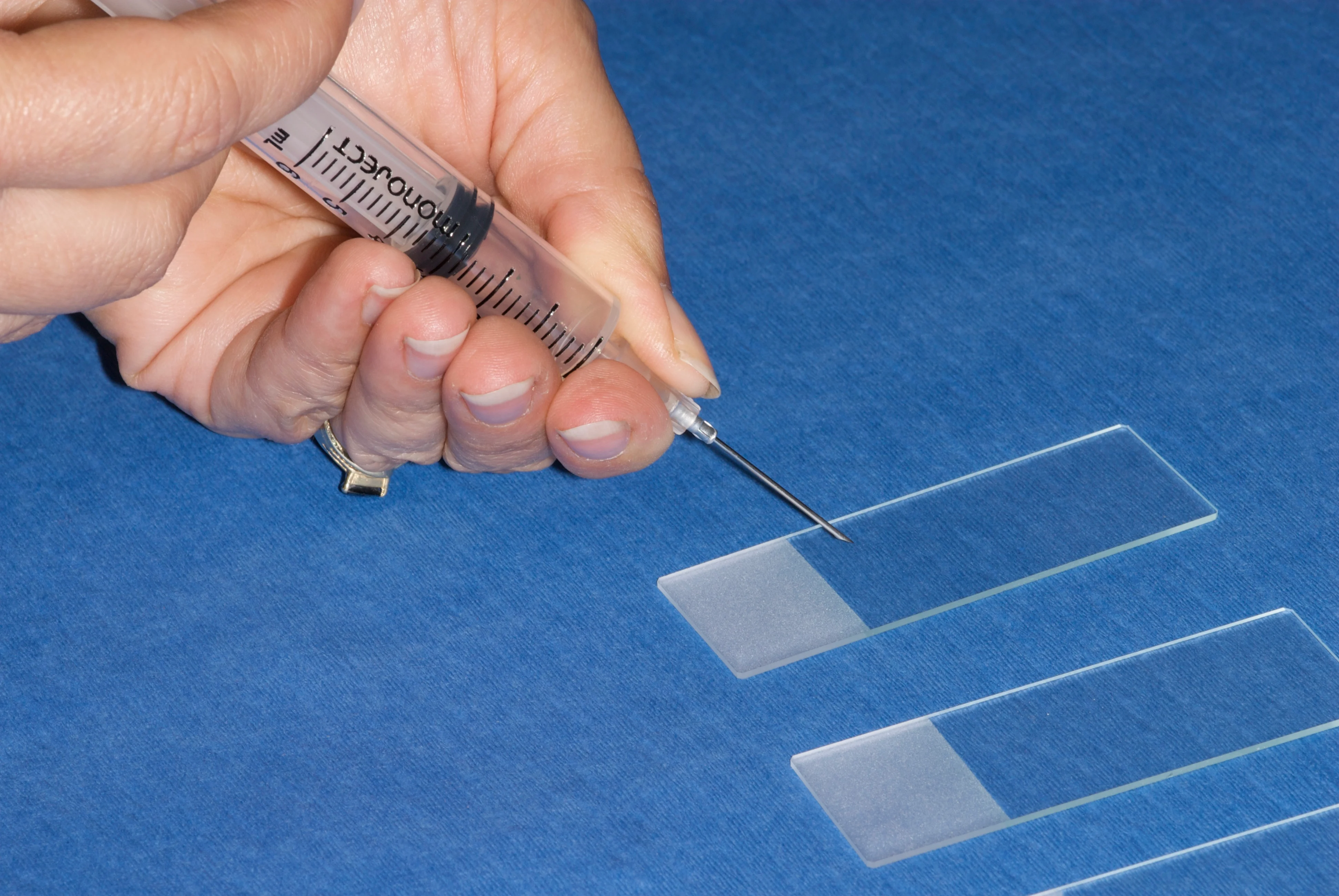
Limited equipment is needed to obtain good cytology samples (Figure 2). However, all the needed materials should be set out and ready to go before the tissue is sampled. Once the sample is obtained, it can rapidly clot, leading to poor smear preparation. The size of the needle is not critical; however, a 25-gauge needle may be best for very small masses, and an 18-gauge is preferable for bony lesions.

Step-by-Step: Fine-Needle Aspiration
Simple FNA (Fenestration)
This technique involves fenestration, or repeated insertions of a needle into the tissue, without a syringe attached.
Step 1
Hold the tissue to be sampled firmly with the nondominant hand. With the dominant hand, insert the needle through the skin and into the tissue. Quickly move the needle back and forth, 4 to 8 times, into the tissue, keeping the needle under the skin at all times. For a large sampling area, redirect the needle in a fan shape during the multiple fenestrations.
Author Insight
Place a finger over the hub of the needle during simple FNA; in the case of a fluid-filled mass, this keeps fluid from squirting out.

Step 2
Withdraw the needle, attach the syringe, and expel the sample onto a slide (see Smearing Techniques, page 64). Before attaching the needle, make sure the syringe has 4 to 6 mL of air in it for rapid use.
True Aspiration
Alternatively, true aspiration can be used for sample attainment.
Step 1
Attach the 6-mL syringe (with the seal broken but all air expelled) to the needle and insert the needle into the tissue. Hold the syringe in the dominant hand.

Step 2
Once the opening in the beveled part of the needle is completely inserted so that negative pressure is obtained with aspiration, draw back the plunger on the syringe 1 to 3 mL and release it rapidly 3 to 5 times. Release the suction on the syringe before withdrawing the needle from the tissue.
Author Insight
Once the needle is inserted, the plunger is pulled back to obtain 2 to 3 mL of negative pressure.

Step 3
Remove the needle from the syringe and aspirate 4 to 6 mL of air into the syringe. It is critical to remove the needle before drawing air into the syringe; if that is not done, the sample will be pulled into the syringe and the amount expelled will be poor. Expel the sample onto a slide (see Smearing Techniques, page 64).

Mast cell tumor at the medial canthus in a Siamese cat; this is one location where needle aspiration may be less irritating and safer than fenestration in an awake patient.
Why Use One Technique Over Another?
Fenestration
The greatest advantage to fenestration is the ease of using only a small needle and rapidly “poking” the tissue several times with only that needle. Handling the syringe and needle and having to pull back on the syringe is much more cumbersome. For very small areas of interest (eg, feline lymph nodes, small masses), the sensitivity of aim is decreased with the increased volume of equipment and distance of the dominant hand from the sampling site. In addition, fenestration involves less blood contamination.
For internal organs, fenestration is generally the preferred technique but is usually performed with the needle attached to the syringe to prevent air from entering a body cavity through an open needle hub.
Aspiration
Aspiration can be advantageous if the mass is extremely firm and does not release cells with the fenestration technique. In those cases, the added force of the negative pressure generated in the syringe may pull firmly attached cells into the needle. Note, however, that most firm masses will exfoliate very well with the fenestration method.
Aspiration is also helpful if the areas to be sampled are extremely sensitive or painful or are near a critical structure that should not be punctured, such as the eyelid margin (Figure 3) or an inflamed digit. Entering the tissue with the needle only once and holding the needle still while aspirating with a syringe can be less uncomfortable than multiple fenestrations; with this process, it is also less likely that the needle will enter a tissue that is contraindicated.
Step-by-Step: Smearing Techniques
The main goal in creating slides for cytologic assessment is to make smears that are thin and evenly distributed without rupturing the cells. It is best to create multiple slides from one FNA procedure—this enables the clinician to stain one with an in-house stain (Diff Quik) for sample assessment and send unstained slides to the cytology laboratory for a Wright-based stain (and potentially other special stains).
The key to getting a thin, even sample is to start with a very small sample to smear. This small amount of tissue can be put on the slides in 1 of 2 ways. With either method, the sample should be expelled onto the slide with the needle bevel pointing down toward the slide and the bevel over the slide near the frosted edge to give the most room for smearing (Figure 4).

Note the position of the needle and needle bevel related to the slide—expel contents near the frosted edge.
Expelling Sample onto Multiple Slides
Step 1
Gently and carefully expel a very little amount of sample onto as many slides as possible (A). Most FNAs will yield enough sample for 4 to 6 slides (B).


Author Insight
The same smearing slide can be used to smear every slide from a sampling procedure of a single site.
Step 2
To create a thin, even, unruptured sample, place the smearing slide crosswise to the sample slide on the tissue sample and gently press it down to flatten the sample.

Step 3
Keeping the slides touching via the same gentle pressure, pull the smearing slide to the end of the sample slide and off (A). If the slides stay flat against each other, the sample will be smooth and even (B).


Step 4
Be careful to avoid having one of the smearing slide edges touches the sample slide with disproportionate pressure; the sample will get dragged and have ruptured areas and areas that are too thick. The slide on the left is the goal of good sample processing. The slide on the right shows the result of unequal pressure on the top slide; note the irregular shape of the smear, with very thin and thick areas.

Author Insight
A new smearing slide must be used for every new sample site to avoid transfer of cells from the smearing slide to a sample slide from a different site.
Complications
Complications associated with FNA of internal organs are rare; hemorrhage from the liver or spleen (A) and pneumothorax (B) in cases of severe lung disease are very unlikely but should be discussed as potential sequelae with owners.
Expelling Sample onto One Slide
Step 1
This technique, which is my preferred one, expels all of the sample onto one slide. Although it sounds confusing at first, it is a rapid and effective technique.

Author Insight
The dominant hand holds the smearing slide the entire time. Use the nondominant hand to pick up the initial sample slide so that a small amount of sample transfers to the smearing slide.
Step 2
The smearing slide is used to barely touch the large sample and pick up a small amount on its underside (A). This leaves you with2 sample slides—the original sample slide with sample remaining and the top smearing slide with a drop of sample on the underside (B).


Step 3
The sample slide is set down and a clean slide picked up in the nondominant hand; the dominant hand continues to hold the smearing slide. The smearing slide now presses down on the clean slide and flattens the sample (see Expelling Sample onto Multiple Slides, Step 1). From here, the sample is smeared (see Expelling Sample onto Multiple Slides, Steps 2 and 3).

Step 4
The smearing slide then picks up another small bit of sample to smear on a new slide. This goes on until only a very small amount of sample is left on the first slide, which is then smeared itself.

Author Insight
The nondominant hand goes back and forth between lifting the sample slide and the new empty slide.
Checking Your Sample
The last step in the process is to stain one slide and scan it under the microscope to verify that the slides are adequate for laboratory submission.