Feline Infectious Peritonitis [2014]
Profile
Definition
Feline infectious peritonitis (FIP), characterized as an immune-mediated pyogranulomatous vasculitis, is a fatal infection caused by virulent feline coronavirus (FCoV).1-3
Related Article: FIP Update: Hope for the Future
Systems
FIP commonly affects abdominal organs.
Thoracic organs, the eyes, and the CNS can be also affected.1,2
Genetic Implications
A polygenic mode of inheritance has been suggested, and heritability is reportedly >50% in some pedigree catteries.4-6
Incidence & Prevalence
FCoV infection is highly prevalent, especially in crowded environments (eg, catteries, shelters).7
Approximately 25% of cats in single-cat households and 75%–90% in multicat households have antibodies to (and are serologically positive for) FCoV.4
Reportedly, 7.8%–12% of FCoV-infected cats may develop FIP.2,4
It has been suggested that FIP mortality may be ~5% in densely populated environments but much lower in households with 1–2 cats.4,7
Geographic Distribution
FIP is found worldwide, as FCoV is ubiquitous among domestic cats.
Wild felid populations may also become infected.1
Signalment
Breed Predilection
Bengals, Abyssinians, Himalayans, Birmans, Rexes, and Ragdolls are reportedly (with some controversy) at higher risk.<sup5,6 sup>
An Australian study found Burmese, Australian Mists, British Shorthairs, and Cornish Rex to be overrepresented and Domestic Shorthairs and Persians underrepresented.8
Variability in the reported breed incidence from country to country suggests susceptibility to disease is more related to families and bloodlines than to breeds.
Age & Range
Most FIP patients are <2 years of age.
FIP has been observed in cats as old as 17 years of age.2,9
Sex
Intact males are at increased risk.7
Spayed females are at decreased risk.7
Causes
Cats can become infected with coronavirus (an RNA virus within Coronaviridae)10 via fecal–oral transmission.
Feline enteric coronavirus (FECV) is an enteric form of FCoV.
It was previously thought that FECV spontaneously mutates in vivo to FIP virus (FIPV), causing FIP.
A more recent hypothesis supports the existence of virulent and avirulent strains of coronavirus in feline populations.11
Both theories may be partially correct.
Risk Factors
Young age
Intact (males)
Immune status
Stress
Recent surgery
Overcrowding
Breed predisposition
Dose and virulence of FECV
Reinfection rate of recovered cats1,7
Pathophysiology
FECV is spread via fecal shedding from the ileum, colon, and rectum.1
Naïve cats can become infected by ingesting FECV while grooming.
Once ingested, FECV replicates in intestinal epithelial cells.
Most cats can eliminate the virus, and a few may become healthy carriers.12
Almost all kittens become infected with FECV at 6–9 weeks of age.
FECV particles can be found in tonsil and small intestinal tissues within 24 hours of ingestion.
Within 2 weeks, the cecum, colon, mesenteric lymph nodes, and liver become infected.
Virus replication can destroy the enterocytes and cause diarrhea.
FECV has systemic and intestinal phases.
FECV generally remains in the enterocytes without causing further illness.
In cases of FIP, FECV strays from the enterocytes, infecting circulating monocytes and tissue macrophages.
Infected macrophages can destroy the virus if they receive the proper immunologic signals. If not, they become incubators.
An immunologically competent host can also attempt to destroy infected macrophages to limit infection.
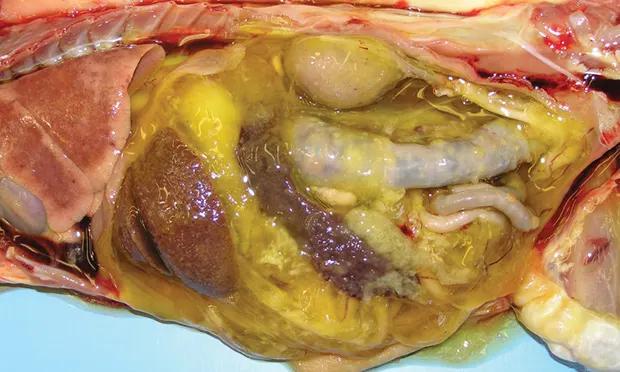
Figure 1. A granulomatous lesion from the liver. Typical liver cells are seen on the left (white arrow). Significant pyogranulomatous inflammation is visualized in the center and to the right of center (yellow arrow; magnification 20×). Courtesy Drs. Dave Getzy & Jeremy Johnson, IDEXX Laboratories
Approximately 10% of cats persistently shed virus, 80% undergo repeated infection, and 10% develop solid immunity.13
FIP lesions can develop secondary to the host’s immune response.
Antibodies are attracted to the virus, complement is fixed, neutrophils and macrophages concentrate at the site, and granulomatous lesions develop (Figure 1).
Lesions may also develop from deposition of circulating antigen–antibody complexes in the vascular walls, causing complement fixation and granulomatous lesions.
As FIP progresses, tissue damage occurs and immune-mediated vasculitis develops, leading to exudate formation.
Eventually, the coagulation system is activated and disseminated intravascular coagulation (DIC) may develop.1
FIP may present as wet (effusive), dry (noneffusive), or a combination of the two.
The dry form results if a partial cell-mediated immune response to FIPV is mounted.
If cellular immunity fails, the patient develops the wet form.7
Although clinically uncommon, both the wet form and the dry form can transform into the other form.1
The dry form appears to be more common in older cats.
Despite many theories on FIP manifestation, individual response to viral infection, genetic predisposition, and environmental factors determine FIP development.7,14
FECV–FIPV Theories
Internal mutation hypothesis
A genetic mutation occurs within the viral population after infection of a susceptible individual, causing the avirulent FECV to become virulent.
FIPV is genetically separate from FECV, and mutations are consistently present in the 3c gene.
However, the genetic differences are small and inconsistent, which is why tests based on viral genetics have failed to consistently distinguish FECV from FIPV.
Rarely, FECV may spontaneously mutate in the enterocytes, changing the virus surface structure.
This enables macrophages and monocytes to phagocytize the virus.
The mutated virus then replicates in local macrophages and monocytes.
Cocirculating virulent and avirulent FCoV strain hypothesis
There are 2 biotypes of FCoV: type I (most common, more likely to cause FIP) and type II (less common).
These viruses use different cellular receptors and have different growth properties; FECV and FIPV come in both biotypes.
FIPV is the virulent FCoV biotype.
FECV is the avirulent FCoV biotype.
Because clinical manifestation of FIPV is caused by the host’s immune response, FIP is not generally transmitted between cats.
Examination & Clinical Signs
Clinical signs vary, depending on the organs affected and FIP form.
History
Young cat from a multicat environment, often recently adopted
Poor doer
Fever (unresponsive to antibiotics)
Lethargy
Decreased appetite or anorexia
Weight loss
Abdominal distention
Dyspnea
Vomiting
Common on Presentation
Fever
Icterus
Ascites (wet form)
Dyspnea from pleural effusion (wet form)
Pericardial effusion (wet form)
Abdominal lymphadenopathy
Thickened intestinal walls
Renomegaly
Uveitis
Keratic precipitates
Retinal changes
Multifocal neurologic signs
Less Common on Presentation
Synovitis
Scrotal enlargement
Skin fragility
Cutaneous nodular lesions
Diagnosis
Definitive Diagnosis
Diagnosis is based on history, clinical signs, laboratory findings (eg, blood tests, fluid analysis), and antibody titers (which should only be used in combination with other diagnostic evidence).<sup3 sup>
Because biopsy results are needed for definitive diagnosis, FIP is seldom confirmed until necropsy.
Antemortem biopsy usually is not routine because of invasiveness, cost, and assumptions based on previous diagnostics, but they can be beneficial in appropriate cases.
Differential Diagnosis
Lymphoma, adenocarcinoma, or other neoplasia
FeLV
FIV
Toxoplasmosis
Heart failure
Liver disease (cholangiohepatitis, cholangitis)
Bacterial peritonitis
Neoplasia
Pleuritis
Laboratory Findings
Mild-to-moderate nonregenerative anemia
Leukocytosis with lymphopenia and neutrophilia
Hyperproteinemia
Hyperglobulinemia (mainly γ-globulins)
Hypoalbuminemia
Albumin:globulin ratio
>0.8 rules out FIP
<0.4 suggests FIP likely
Azotemia
Elevated alanine aminotransferase and alkaline phosphatase
Hepatic hyperbilirubinemia
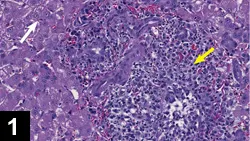
Characteristics of effusion (Figure 2)
Light to dark yellow (Figure 3)
Mucinous
Protein, 3.9–9.8 g/dL, mostly γ-globulins
Macrophages, neutrophils, and lymphocytes on cytology; low-to-moderate cellularity (<5000 cells/µL)
Specific gravity 1.017–1.047
Cerebrospinal fluid cytology
Protein >200 mg/dL
WBC >100 cells/μL (mostly neutrophils, lymphocytes, macrophages)
Normal findings on laboratory tests do not rule out FIP
_Figure 2. Cytology of abdominal effusion from a cat with FIP. Cellularity is composed of 70%–80% neutrophils and 20%–30% mononuclear cells. Many of the neutrophils occur in clumps or groups (dashed arrow) and are slightly lytic or degenerated. Mildly vacuolated macrophages (solid arrow) are present. The background is heavy and amphophilic with an eosinophilic granularity. Numerous protein crescents (dotted arrow) are also present. (Magnification 50×). Courtesy Dr. Dean L. Cornwell, IDEXX Laboratories.
Figure 3. Lungs and abdominal viscera of a cat with FIP (effusive). Note the punctate to coalescing fibrinous plaques covering the serosal surfaces of the lungs and visceral organs. Classic, yellow, fibrinous effusion is also visualized. Courtesy Drs. Steve Rushton & Jeremy Johnson, IDEXX Laboratories._
Imaging
Radiography
Nonspecific changes consistent with effusion in abdominal and/or thoracic cavities can be seen.
If there is pulmonary involvement, patchy opacities in the lungs may be visualized.1
Radiographic findings may be unremarkable.
Ultrasonography
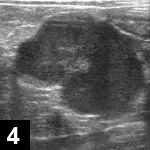
Mesenteric lymphadenopathy (Figure 4)
Renal
Renomegaly
Irregular renal contour
Hypoechoic subcapsular echogenicity (Figure 5)
Ascites/retroperitoneal effusion
Intestinal changes
Wall thickening
Presence of a solitary mass at the ileocecocolic junction
Other differentials for a solitary mass include fungal granuloma, lymphoma, and adenocarcinoma.
No pathognomonic changes associated with FIP are seen on ultrasonography; normal abdominal ultrasound findings cannot rule out FIP.
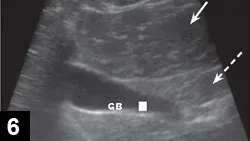
Liver and spleen often appear normal (Figure 6).15
Figure 4. Abdominal ultrasound from a cat with FIP (noneffusive). A large, well-defined, irregularly marginated, 3-cm hypoechoic mass in the midabdomen (most likely a mesenteric lymph node) is present.
Figure 5. Abdominal ultrasound from a cat with FIP (noneffusive). A kidney with hypoechoic subcapsular echogenicity (solid arrow) is present. The renal cortex (dashed arrow) and renal medulla (dotted arrow) are also seen.
Figure 6. Normal liver (solid arrow) in a cat with FIP. The gallbladder (GB) and falciform fat (dashed arrow) are also apparent.
MRI & CT
Nonspecific changes associated with inflammation of the CNS
May assist in localization of lesions
May help differentiate FIP from other infectious neurologic diseases.7,16
Hydrocephalus in previously neurologically normal cat may indicate neurologic FIP.1
Other Diagnostics
Coronavirus Antibody Titers
May be helpful but results need careful evaluation
FIP cannot be diagnosed by positive titer or ruled out by negative titer.
Titers may drop terminally, because antibodies bind to large amounts of the virus (~10% of cats with FIP have negative titers).17
Many healthy cats may be antibody positive and never develop FIP, regardless of titer level.
Results from different laboratories cannot be compared if they use different antigens.
Extremely elevated titers may suggest FIP if signalment, examination, laboratory testing, and other diagnostic test results are also compatible.1,2,17
Rivalta Test
A relatively inexpensive, simple test easily completed in house (has some limitations)
Detects effusion high in protein, fibrin, and inflammatory mediators
Helps differentiate FIP fluid from fluid of other disease processes
Bacterial peritonitis and lymphoma may cause a false-positive result.7,17
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Detects active coronavirus infection
Impossible to distinguish between virulent and avirulent coronavirus biotypes
Performed on sera, feces, effusions, and biopsy samples or fine-needle aspirates of affected organs
Because false-negative and positive results can occur, evaluating results in conjunction with other diagnostic tests and clinical findings is important.1,2,10,17,18
Auburn University offers a quantitative test that detects FIP mRNA and is highly sensitive and specific.
Detects and quantifies replicating FECV in fluid specimens, tissue samples (from biopsy or aspiration), blood, and feces
Histopathology
The gold standard for diagnosing FIP
A lesion with perivascular granulomatous to pyogranulomatous inflammation and vasculitis is highly suggestive of FIP (Figure 1).10
Immunostaining (Immunofluorescence/Immunohistochemistry)
Helpful if highly suggestive lesions are not seen on histopathology
Detects coronavirus antigen in macrophages
Cannot differentiate between virulent and avirulent coronavirus, but if there is positive staining in macrophages in granulomatous lesions or effusions, it is considered diagnostic for FIP10
Treatment
Diagnosing FIP is imperative, as it is fatal once clinical signs develop.
If FIP is confirmed, euthanasia should be considered.
Supportive medical management
Corticosteroids: suppress inflammation and immune response
Controlled studies are lacking.19
Chlorambucil or cyclophosphamide: used in combination with corticosteroids
Controlled studies are lacking.2
Feline interferon-ω: an antiviral that has not shown benefit (not available in North America)10
Polyprenyl immunostimulant: an immunostimulant
Limited data but may control dry FIP; further studies needed.10,20
Pentoxifylline: may help reduce vasculitis
Recent study showed no increase in survival time or quality of life21
Thromboxane synthetase inhibitor (ozagrel hydrochloride): inhibits platelet aggregation
Improvement of clinical signs seen in 2 cats2
Controlled studies are lacking.1,22
Available in Japan and is available in United States for research purposes only
Other supportive therapy
Fluid therapy
Cats with effusion may benefit from fluid removal.
Broad-spectrum antibiotics if secondary infections suspected
Nutritional support
In General
Relative Cost
$$$$$
Prognosis
Poor to grave
Survival time is reportedly 3–200 days, but all cats ultimately die once clinical signs develop.1
Prevention
Vaccination
Modified live, nonadjuvanted, and intranasal
Vaccination against FIP is generally not reliable nor recommended by the American Association of Feline Practitioners.
May be of benefit in coronavirus-negative kittens
Vaccination of coronavirus-antibody–positive cats or cats living with another cat confirmed or suspected to have FIP is not recommended.10,23
Management Strategies
The only way to prevent FIP is to prevent FECV infection.1,2,7,10
Hygiene and keeping litter boxes clean are important.
Although it cannot reliably prevent infection, litter boxes should not be kept in the same room as food bowls.2
An abundance of cats in individual households should be avoided; some recommend that cats be kept in groups of 3 or fewer per room.2
Cats should be housed individually in a shelter to avoid overcrowding.2
If a cat with FIP has been euthanized, 3 months should pass before introducing another cat to the household; this assumes the safest possible course, as coronavirus is not environmentally persistent and can sometimes degrade within days.1
However, if there are other cats in the household, most likely they are already infected with FECV.
If coronavirus is not endemic in a cattery, an infected cat should not be introduced.
RT-PCR testing can be used to detect FECV in the feces, but multiple samples must be tested over time to establish infection status.1,2
After 5 consecutive months of negative fecal RT-PCR test results, a cat may be considered FECV negative.24
FCoV = feline coronavirus, FIP = feline infectious peritonitis, FECV = feline enteric coronavirus, FIPV = FIP virus, RT-PCR = reverse transcriptase-polymerase chain reaction
_BRISA HSIEH, DVM, is a second-year small animal internal medicine resident at Gulf Coast Veterinary Specialists in Houston, Texas. Previous, she completed a one-year rotating internship at Veterinary Emergency Referral Group in Brooklyn, New York. She earned her DVM from Kansas State University.
DEREK P. BURNEY, DVM, PhD, DACVIM, is affiliated with Veterinary Specialists of North Texas in Dallas. His interests include feline medicine and parasitology. Dr. Burney completed a one-year internship at Auburn University followed by a residency in small animal internal medicine and a PhD in molecular parasitology at Colorado State University. He earned his DVM from Texas A&M University._