History & Signalment
Pumpkin, a 7-year-old, 5.9-lb (2.7-kg) spayed domestic shorthair cat, was presented to the emergency clinic after 1 week of hiding in her foster home, where she had been placed immediately after being surrendered to an animal shelter. The foster caregiver was unable to determine whether Pumpkin had been eating or drinking; however, the evening prior to presentation, the caregiver retrieved Pumpkin from her hiding place and offered her wet and dry food, but she showed no interest in eating. No history prior to surrender to the shelter was available.
Physical Examination
On physical examination, Pumpkin was quiet, alert, responsive, and fearful. Examination results revealed BCS of 2/5, dilated pupils, tachycardia (heart rate, 208 bpm), urine staining on the pelvic limbs, icteric skin and sclerae, and icteric and tacky mucous membranes. Dehydration was estimated to be ≈6% to 7% based on prolonged skin tenting, capillary refill time was <2 seconds, and there was no physical evidence of enophthalmos.
Diagnostics
CBC and serum chemistry profile (Table 1) revealed mild, normocytic, normochromic, nonregenerative anemia; increased ALT; increased ALP; hyperbilirubinemia; and hyperglycemia. Protein and electrolytes were within normal limits. Coagulation assay and urinalysis yielded no significant abnormalities; urine specific gravity was 1.041.
Table 1: Selected Values From CBC & Serum Chemistry Profile
Abdominal ultrasonography revealed an enlarged liver with rounding of the caudal ventral margin. The hepatic parenchyma was hyperechoic and hyperattenuating, suggestive of lipid deposition. A fine-needle aspirate of the liver revealed abundant hepatocytes with marked vacuolation consistent with lipid (Figures 1 and 2).
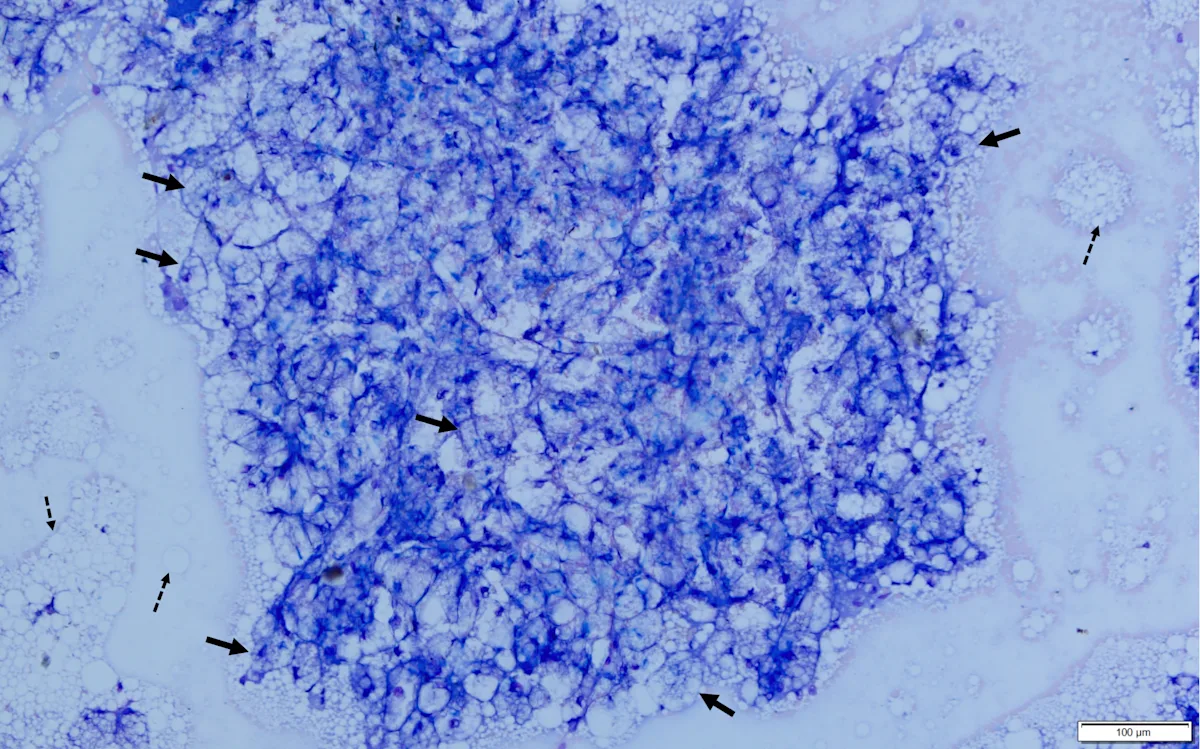
Fine-needle aspirate from the liver of a cat. A cohesive sheet of numerous hepatocytes (solid arrows) can be seen. Cytoplasm of almost all hepatocytes is markedly distended with large (ie, macrovesicular) and small (ie, microvesicular) clear vacuoles that often obscure the nucleus and make observation of hepatocytes difficult. Numerous free lipid droplets are present in the background (dashed arrows). Modified Wright’s stain, 200× magnification
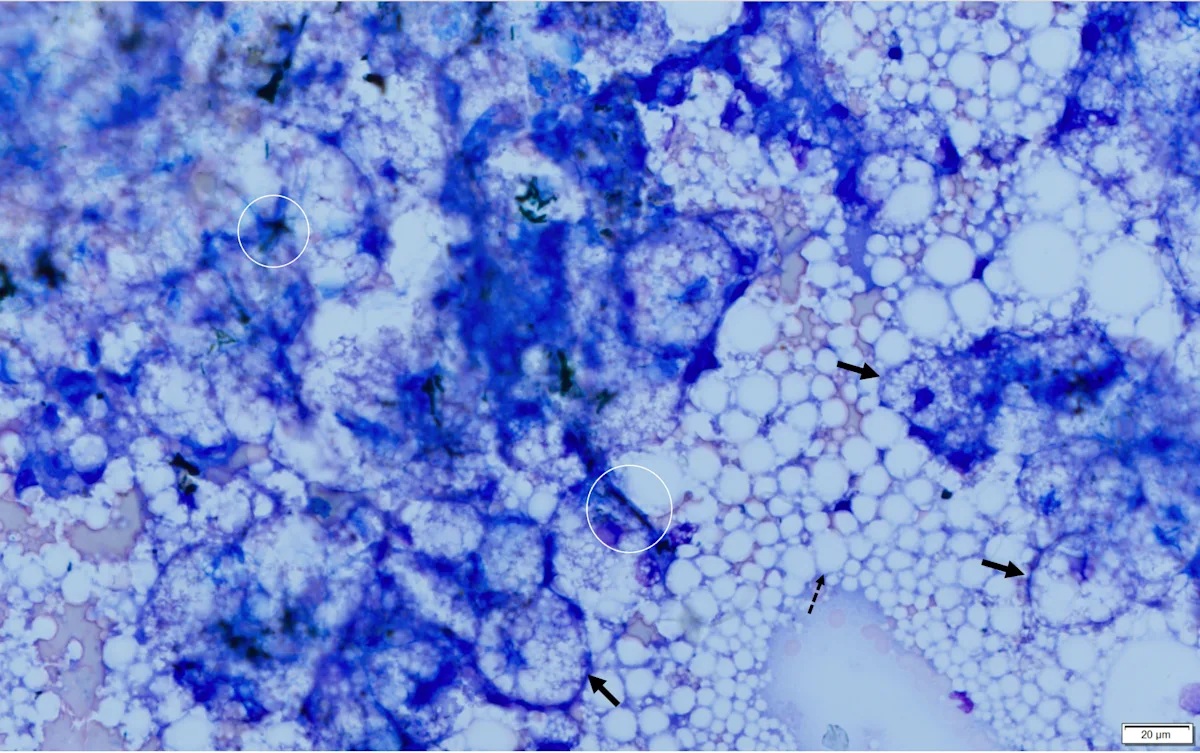
Fine-needle aspirate from the liver of a cat. A cohesive sheet of hepatocytes (solid arrows) can be seen. Cytoplasm of hepatocytes is markedly distended with large (ie, macrovesicular) and small (ie, microvesicular) clear vacuoles that make observation of hepatocytes difficult. Darkly pigmented material (bile casts; circles) can be seen between cells, indicating cholestasis. Numerous free lipid droplets (dashed arrow) are also present in the background, but although this finding is typical with hepatic lipidosis, it is not diagnostic, as lipid must be within the cells. Modified Wright’s stain, 600× magnification
No underlying disease was identified, thus primary hepatic lipidosis due to the stress of being rehomed was assumed.
Diagnosis: Hepatic Lipidosis
Treatment & Outcome
Immediate hospitalization with aggressive therapy was recommended but declined because of financial constraints. A fluid bolus (0.9% sodium chloride, 100 mL SC), vitamin K1 (1 mg/kg SC), and maropitant (1 mg/kg SC) were administered. Pumpkin was discharged with instructions to be kept in an enclosed area (eg, large crate, bathroom) to prevent hiding during the night, given water but not food, and returned the following morning for placement of an esophagostomy feeding tube (E-tube).
For placement of the E-tube, Pumpkin was anesthetized with butorphanol (0.3 mg/kg IM), induced with propofol (4 mg/kg IV), and maintained on isoflurane (1.5%-2%) with oxygen. She was placed in right lateral recumbency, the left lateral midcervical area was aseptically prepared, and an E-tube was placed. The E-tube was secured via finger-trap sutures and capped, and the cervical area was wrapped to allow access for monitoring. Recovery from anesthesia was uneventful.
The resting energy requirement (RER) was calculated with the following equation and determined to be 183 kcal/day.a
RER kcal/day = 70 × (optimal body weight in kg)0.75
A commercial therapeutic critical care diet mixed with room temperature water to produce a 1 kcal/mL slurry was administered over 20 to 30 minutes every 6 hours for the first 3 days, then every 8 hours starting on day 4. The E-tube was flushed with room temperature water before (5 mL) and after (10 mL) each feeding. On the first day of treatment, 10% of the RER (ie, 183 kcal/day) was given across the 4 meals (ie, 18.3 mL of the 1 kcal/mL slurry). Over the following 9 days, the ration was increased by 10% each day until the RER was reached and maintained. This frequency continued until Pumpkin was able to eat on her own. Details of Pumpkin’s tailored feeding plan can be seen in Table 2.
Table 2: Tailored Patient Feeding Plana
a Food was offered before every E-tube feeding.
Lactulose (0.5 mL PO every 6 hours for 3 days, then every 8 hours) was given to help prevent clinical signs of hepatic encephalopathy. After ≈2 to 3 weeks, Pumpkin began to show interest in food. The volume consumed was subtracted from the amount administered through the E-tube. After an additional 2 to 3 weeks, Pumpkin was able to eat the complete RER on her own, lactulose was discontinued, and the E-tube was removed after 4 consecutive days of eating.
Three weeks following E-tube removal, Pumpkin was adopted by the foster caregiver and was reported to be doing well at the 3-month postadoption follow-up.
Treatment at a Glance
Polyionic fluid therapy without lactate or dextrose is recommended.1
Electrolytes should be monitored and derangements addressed.2
Enteral nutritional support is the key component of treatment.1-5
Force feeding can result in food aversion or aspiration pneumonia and is not recommended.2,5
Cats with hepatic lipidosis generally require nasogastric (in-hospital feeding only), esophagostomy, or gastrostomy tubes.2 An E-tube is preferred for home feedings.
A slurry diet that consists of high protein, moderate fat, and minimal carbohydrates is crucial.3
Feeding should be initiated slowly in small portions to prevent refeeding syndrome.3
Appetite stimulants are not recommended.1,3
Nutritional support may be prolonged and requires significant client involvement.3
Recurrence of disease is rare in cats that survive.5
Discussion
Feline hepatic lipidosis (FHL) is the most commonly diagnosed hepatobiliary disease in cats and was previously thought to be idiopathic but is now known to be related to a negative energy balance. Pathogenesis has yet to be determined.1-5 FHL can affect cats of any breed, age, or sex, but middle-aged cats with a high BCS or history of obesity and female cats are overrepresented.1,2
Background
Cats are obligate carnivores and require a high-protein diet with essential amino acids for cellular energy metabolism. Metabolic derangements can rapidly develop when cats become anorexic or hyporexic.1,2 As the energy balance shifts toward negative, lipase activity is stimulated in the peripheral fat to release fatty acids into the bloodstream; these are absorbed by the liver, oxidized, and either incorporated into very-low-density lipoproteins (VLDLs) or re-esterified into triglycerides for intracellular storage. In anorexic cats, most triglycerides are stored in hepatic vacuoles because of the limited capacity of the oxidative system and redistribution through VLDLs.1 Excessive accumulation of triglycerides results in hepatocyte enlargement, increased liver weight, and changes to the overall liver architecture, resulting in cholestasis, icterus, reduced cellular capacity for metabolism, and hepatic failure.1-3 Cytologic assessment of cats with FHL has shown evidence of lipid vacuolation in >80% of hepatocytes.1,2
Primary Versus Secondary Hepatic Lipidosis
FHL can be primary or secondary. Primary FHL occurs when an extrinsic factor (eg, unpalatable food, environmental stress, food insecurity) leads to insufficient caloric intake. Secondary FHL represents 95% of cases and results from a concurrent, progressive disease (eg, neoplasia, diabetes mellitus, inflammatory conditions, renal disease) that subsequently leads to anorexia.2
Presentation
Clinical signs include dehydration, nausea, ptyalism, vomiting, icterus, rough hair coat, lack of grooming, constipation, diarrhea, and a history of anorexia or weight loss.2 Mentation may be altered depending on disease severity, especially if hypokalemia or hyperammonemia and hepatic encephalopathy are present. Cats with FHL are often hyperglycemic because of insulin resistance and increased production of counterregulatory hormones.2 Hypoglycemia in these cases is indicative of severe disease, suggesting end-stage liver failure.
Diagnostics
CBC parameters are often within normal limits. Nonspecific findings (eg, mild nonregenerative anemia, mild leukocytosis) may be present but often depend on the underlying cause. Thrombocytopenia is not expected in cats with primary FHL unless disseminated intravascular coagulation is present.2
Clinical bleeding is common in cats with FHL, especially during IV catheterization, blood collection, liver biopsy, and feeding-tube placement.2 Vitamin K-dependent coagulation factors (ie, II, VII, IX, X) are produced in the liver as nonfunctional factors that require the presence of vitamin K for activation. Although vitamin K is a fat-soluble vitamin, stores in the body are minimal, and dietary intake with intestinal absorption is required. Hepatobiliary disease can result in vitamin K deficiency secondary to inadequate digestion and absorption of fat. With vitamin K deficiency (or antagonism, as can occur with warfarin), the nonfunctional vitamin K-dependent coagulation factors (referred to as proteins induced by vitamin K absence or PIVKAs) build up in circulation. One study showed 75% of cats with FHL had increased concentrations of PIVKAs,1 suggesting that 75% of cats with FHL are vitamin K deficient. Thus, prothrombin time and activated partial thromboplastin time are frequently increased in patients with FHL.2
Diagnosis
Presumptive diagnosis is often made based on patient history, physical examination, blood work, and imaging.2 Definitive diagnosis is usually based on cytologic evaluation of a fine-needle aspirate. Fine-needle aspiration can often be performed without general anesthesia and with a lower risk for hemorrhage than a larger tissue biopsy; however, a larger tissue biopsy may be needed if fine-needle aspiration does not yield a diagnosis.2
Treatment
Treatment is not complicated, but early initiation and close monitoring are more likely to result in favorable outcomes. Serum chemistry profile, urinalysis, CBC, and coagulation assessment should ideally be performed for all suspected cases of FHL and can help direct treatment and identify condition severity and potential underlying causes.2,5
If FHL is suspected, prophylactic vitamin K1 (0.5-1.5 mg/kg IM or SC) should be administered at admission to decrease risk for bleeding during procedures.5 Rehydration (ideally, IV) and electrolyte supplementation should be performed as needed. Fluids without lactate or dextrose can negatively affect hepatic lipid transport and are therefore recommended.1 Once hydration status and electrolyte abnormalities have been addressed, the patient should be given a high-quality diet (ie, 33%-45% protein, moderate fat content, and low carbohydrate content to prevent GI adverse effects), likely through a nasoesophageal (in-hospital feeding only), esophagostomy (authors’ preference for home feeding), or gastrostomy tube to ensure adequate caloric intake.3 Food reintroduction that occurs too quickly can precipitate refeeding syndrome and result in severe, life-threatening electrolyte derangements. Initial trickle feeding via CRI may be beneficial.3 Appetite stimulants are not recommended because of unpredictable results, alterations of hepatic metabolism, and masking effects.1,3
Feeding should be initiated with no more than 20% of the ideal RER divided into 4 to 5 small meals or given via CRI, then increased by 10% every 24 hours while monitoring for signs of retching or gulping.3 The authors’ preference is to begin with 10% of the ideal RER to minimize the chance of retching or gulping. If retching or gulping occur, the volume of food should be reduced by 50% for the following 12 hours.3 Antiemetics may be administered, but the underlying cause of retching and/or gulping should be investigated.3 Food should be warmed to between room (minimum) and body (maximum) temperature and offered before every feeding, with any amount eaten subtracted from that feeding’s volume. Heating food in a water bath rather than a microwave can help ensure an even temperature without overheating. Before feeding, the tube should be slowly flushed with 5 mL of room temperature water to ensure it is not clogged. To allow gastric expansion, food should also be infused slowly. Following feeding, a slow, room temperature water flush (≈10 mL) is needed to prevent clogging of the feeding tube.3 In the authors’ opinion, each feeding should be completed over a minimum of 20 to 30 minutes. Because Pumpkin was responding well after several days of feeding every 6 hours with careful monitoring for retching or gulping, feeding frequency was reduced to every 8 hours in an attempt to ensure caregiver compliance.
In a study investigating the effects of diacylglycerol O-acyltransferase 1 inhibitors on triglyceride accumulation in hepatocytes during FHL, the drug T863 was found to reduce triglyceride accumulation by 52%.6 Although this study was performed on feline liver organoids ex vivo, potential future therapeutic options may be possible.
Prognosis
Without treatment, the survival rate is low (5%-20%). With treatment, the survival rate increases to 50% to 60% for secondary FHL and 80% to 88% for primary FHL.2-4
Prognosis is determined by the presence or absence of underlying disease and how rapidly enteral nutritional support is initiated. Clients should be counseled that tube feeding requires significant involvement and may be prolonged (usually over 3-6 weeks).3 FHL recurrence is rare in cats that survive.5
Take-Home Messages
FHL is the excessive accumulation of triglycerides within hepatocytes during times of negative energy balance and is the most common feline hepatobiliary disease.
FHL is a life-threatening disease that requires prompt treatment.
Middle-aged, overweight cats or cats with a history of obesity are overrepresented, but FHL can affect any breed, age, or sex.1,2
Early intervention is critical for successful treatment.
Treatment success rate is high, especially in cases of primary FHL.
a Resources for determining RER based on optimal patient body weight are available.