Breed-Specific Amyloidosis: Familial Shar-Pei Fever
Jenessa A. Winston, DVM, PhD, DACVIM, North Carolina State University
Profile
Definition
In familial renal amyloidosis of shar-peis, deposition of amyloid can progressively disrupt normal renal architecture, leading to chronic kidney disease (CKD).
Amyloidosis is the extracellular deposition of fibrils formed by polymerization of proteins with a beta-pleated sheet conformation.
Reactive amyloidosis secondary to chronic infectious and noninfectious inflammatory disease and neoplasia is the most common form in animals.
Renal amyloidosis can result in CKD, proteinuria, and nephrotic syndrome.
Many shar-peis will have fever and swelling of the tibiotarsal joints (also called shar-pei fever or shar-pei swollen hock syndrome) before development of renal amyloidosis.
The cause of this syndrome in shar-peis is unknown.
Although this disease is considered genetic, not all shar-peis with the trait will develop renal amyloidosis (see Genetic Implications).
Not all shar-pei fever patients will have renal amyloidosis.
Systems
Renal dysfunction is the most common; however, other organ systems can be affected by amyloid deposition.
Genetic Implications
In shar-peis, this is an autosomal recessive trait.
Incidence & Prevalence
Renal amyloidosis is estimated to occur in 23% of shar-peis in the United States.
True prevalence is unknown.
Signalment
Breed Predilection
Shar-peis are predisposed.
Familial renal amyloidosis has also been reported in beagles, English foxhounds, collies, Walker foxhounds, and Abyssinian and Siamese cats.
Age & Range
Age of onset of clinical signs is typically 1–6 years (mean, 4.1 years).
Sex
More common in female than male dogs (female:male ratio, 2.5:1)
Pathophysiology
Amyloid A protein, formed by the polymerization of the amino acid terminal portion of serum amyloid A (SAA) in response to inflammatory cytokines, is the primary protein involved in reactive amyloidosis.
Affected shar-peis have increased serum concentrations of interleukin-6, a cytokine that stimulates synthesis of SAA and the release from hepatocytes.
Other cytokines (eg, tumor necrosis factor-α, interleukin-1β) are also involved.
These cytokines initiate the acute phase response characterized by fever, hepatic production of acute proteins (including SAA), and mobilization of neutrophils.
Amyloid deposition disrupts normal tissue architecture and can cause organ failure.
In shar-peis, amyloid deposition can occur in the kidneys, liver, spleen, pancreas, adrenal glands, thyroid glands, myocardium, prostate, lymph nodes, and GI tract.
Most do not show signs of organ dysfunction other than kidney or hepatic disease.
Renal amyloidosis in other canine breeds can lead to marked proteinuria.
Only 25%–43% of affected shar-peis have proteinuria.1
Nephrotic syndrome—characterized by marked proteinuria, hypoalbuminemia, hypercholesterolemia, and edema—can be present.
Some affected dogs are at increased risk for thromboembolic disease, in part because of loss of antithrombin through the affected glomerulus.
A similar syndrome of fever and synovitis called familial Mediterranean fever occurs in humans.
History & Physical Examination
Intermittent episodes of fever ± joint swelling or pain
Episodes often precede amyloidosis, although these episodes may not be detected.
At initial presentation, intermittent high fever (ie, 103°F–107°F) and joint swelling (eg, tibiotarsal joints) that resolve ± treatment may be present.
Affected patients may appear normal if fever and joint swelling are not present.
Marked CKD may result in oral ulceration, uremic breath, and dehydration.
Nephrotic syndrome may result in ascites, SC edema, or both.
Acute onset of respiratory distress, tachypnea, or pelvic limb paresis may indicate thromboembolic disease.
Jaundice occurs if hepatic amyloidosis is present.
Hepatic amyloidosis has been reported in ~11% of cases.2
Clinical Signs
Signs include polydipsia, polyuria, anorexia, vomiting, dehydration, weight loss, weakness, and lethargy.
Diagnosis
Definitive
Renal biopsy specimen should be obtained from the renal cortex to reduce complications (eg, hemorrhage, infarction).
Because amyloid deposits are often limited to the medulla, the diagnosis may be unobtainable on renal biopsy; however, medulla biopsies are not recommended because of risk for complications.
Approximately 64% of shar-peis will have glomerular involvement.
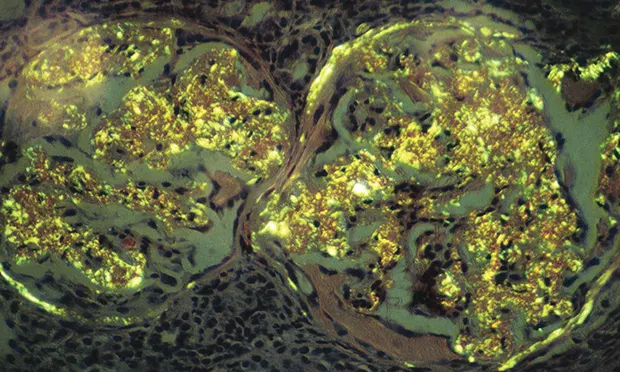
Staining with Congo red (see Figure 1, Renal biopsy specimen stained with Congo red showing typical birefringence of glomerular amyloid deposits. Image courtesy S.P. DiBartola)
Light microscopy discloses amyloid deposits in various shades of red.
Polarizing microscopy discloses amyloid deposits in an apple green birefringence.
Amyloid deposition is confirmed by decolorization of Congo-red–stained deposits by potassium permanganate oxidation.
If intermittent fever and joint swelling precede onset of CKD signs in a shar-pei, renal biopsy is not recommended.
Treatment of presumed amyloidosis should be initiated.
Aspirates from other organs (ie, liver, spleen) can be obtained if positive staining with Congo red is documented.
Differentials
Joint disease
Polyarthritis (ie, immune mediated, bacterial, viral, fungal)
Lyme disease, especially in endemic areas
Ehrlichiosis
Vaccine reaction
Renal amyloidosis
Other glomerular diseases

Laboratory Findings
CBC
Nonregenerative, normocytic, normochromic anemia, secondary to CKD
Serum biochemistry profile
If renal amyloidosis is present:
Azotemia
Hyperphosphatemia
Metabolic acidosis
Hypoalbuminemia
Hypercholesterolemia
Hyperglobulinemia
If hepatic amyloidosis is present:
Increased alkaline phosphatase, alanine transaminase, and aspartate transaminase activities
Hyperbilirubinemia
Urinalysis
Proteinuria is considered the hallmark of glomerular disease but is variable (25%–43%) in shar-pei fever because amyloid deposition occurs mainly in renal medulla.
Urine protein:creatinine (UP:C) should be measured if proteinuria is present.
UP:C >0.5 is considered abnormal.
Isosthenuria
Systemic hypertension
Imaging
Abdominal radiography can show hepatomegaly and relatively normal kidneys.
Abdominal ultrasonography can show hyperechoic renal cortex, decreased corticomedullary distinction, and a hypoechoic liver with rounded edges.
Other diagnostics:
Assessment of hypercoagulability
Coagulation panel
Antithrombin or antithrombin III concentrations
Thromboelastography
Postmortem findings
Confirmation of renal (or other) amyloidosis
Lugol’s iodine can be applied to the cut surface of the kidney, which will yield bluish-black dots within the tissue representing amyloid deposits.
Reactive amyloidosis can be confirmed by decolorization of Congo-red–stained amyloid deposits by potassium permanganate oxidation.
Treatment
Medical
Initial treatments (see Table)
Supportive care as indicated (eg, NSAIDs) to reduce pain and fever and maintain hydration.
Colchicine
Colchicine can impair release of SAA from hepatocytes by binding to microtubules, which will prevent secretion; this may also prevent production of amyloid-enhancing factor.
Colchicine should be initiated after 2 episodes of fever and joint swelling and after other causes of polyarthritis have been excluded; this can prevent further amyloid deposition.
Colchicine will not eliminate amyloid that has already been deposited; if azotemia is present, colchicine may not reverse existing organ damage.
Therapy is lifelong, independent of persistent fever or swollen joints.
Adverse effects of colchicine include vomiting and diarrhea.
With long-term administration, bone marrow suppression and hypertension are noted.
Dimethyl sulfoxide (DMSO)
Treatment is controversial; there is no proven clinical benefit to date.
DMSO does not appear to solubilize amyloid fibrils; any benefit may be related to the antiinflammatory properties of DMSO.
Enalapril or benazepril
Angiotensin-converting enzyme (ACE) inhibitors for reducing proteinuria
Low-dose aspirin or clopidogrel
May decrease the frequency of thromboembolic disease
Should be started if serum albumin <2.5 g/dL
Aspirin should not be administered if the patient is receiving other NSAIDs.
Antihypertensive agents
Additional agents (eg, amlodipine) should be started if persistent hypertension is present (systolic blood pressure >170 mm Hg) after enalapril or benazepril initiation.
Nutritional
A diet formulated for dogs with renal disease is indicated.
Ensure adequate caloric intake.
Malnutrition is a major cause of morbidity and mortality in shar-peis with CKD.
Additional supplementation with omega-3 fatty acids may be beneficial.
Contraindications
Renal transplantation
Amyloid is likely to deposit in transplanted organs.
Follow-up
Patient Monitoring
UP:C, urinalysis, serum albumin concentration, serum creatinine concentration, and body weight should be monitored monthly when adjustments to therapeutic plan are made.
If a patient presents with fever only, consider monitoring with urinalysis and measuring serum creatinine concentrations q3mo.
Clients can monitor their dog’s body temperature to document febrile episodes.
Response to therapy
50% reduction of proteinuria (based on UP:C) without increase in serum creatinine
Combination of 3–5 pooled urine samples for UP:C evaluation is ideal.
If systemic hypertension is present, blood pressure should be rechecked q3mo until stable.
More frequent monitoring is required if unregulated hypertension is present.
Once patient is stable, parameters can be monitored q3mo.
In General
Relative Cost
Shar-pei fever with renal amyloidosis may be costly because of lifelong medications, supportive care, hospitalization, and diagnostic monitoring: $$$$$
Prognosis
Poor to guarded
Optimal treatment is unclear, but early intervention with colchicine therapy may improve prognosis.
CKD = chronic kidney disease, SAA = serum amyloid A, UP:C = urine protein:creatinine
Editor’s note: This article was originally published in September 2013 as “Familial Shar-Pei Fever.”