Emerging Molecular Tests for Differentiating Inflammatory & Neoplastic Lymphocytes in the Intestinal Tract
Timothy M. Fan, DVM, PhD, DACVIM (Oncology, Internal Medicine), University of Illinois at Urbana–Champaign
In the literature
Joos D, Leipig-Rudolph M, Weber K. Tumour-specific microRNA expression pattern in canine intestinal T-cell-lymphomas. Vet Comp Oncol. 2020. https://doi.org/10.1111/vco.12570
The Research …
The intestinal tract is a highly specialized organ responsible for nutrient digestion and absorption. In intestinal tissue, several cell types (ie, epithelial, mesenchymal, hematopoietic) contribute to preserving normal peristaltic functions and maintaining competent physical barriers to luminal microorganisms. Lymphocytes reside in the submucosal layer of the intestines and are normally involved in immune surveillance, providing a line of defense against luminal pathogens. However, this same population of lymphoid cells can give rise to diverse inflammatory or neoplastic pathologies.
In dogs, 2 notable intestinal diseases include T-cell–rich lymphoplasmacytic inflammatory bowel disease (IBD) and T-cell intestinal lymphoma (LSA).1-3 Because both pathologies have a population of predominately CD3+ T lymphocytes, differentiating between these clinical entities can be difficult, and definitive diagnosis may require a panel of tests, including histology, immunohistochemistry, and molecular profiling through PCR for antigen-receptor rearrangements.4 Identifying complementary surrogate biomarkers, which are reliable and aid in distinguishing inflammatory from neoplastic lymphoid pathologies, could advance veterinary medicine.
MicroRNAs are noncoding RNA sequences found in the genome that regulate translation of proteins following gene transcription. Based on their mechanism of action, microRNAs can control cell population phenotypes by regulating the expression of different signaling pathways that influence biologic behaviors.5 Pathologies that may be phenotypically similar or overlapping (eg, lymphoplasmacytic IBD, intestinal T-cell LSA) could have differential microRNA expression patterns, allowing for these clinically similar diseases to be distinguished from one another based on their microRNA profile.
This study examined the microRNA expression patterns derived from full-thickness intestinal biopsies collected from normal dogs and from dogs that had a confirmed diagnosis of T-cell–rich lymphoplasmacytic IBD or T-cell LSA. Out of 183 mature microRNA candidates, 12 specific microRNAs were selected based on their robust and highly differential expressions across groups (normal, IBD, LSA), as well as on their predicted functional role in pathologic lymphoid biology.
When evaluated across 8 normal, 8 IBD, and 8 T-cell LSA intestinal samples, the 12 microRNA panels produced differential patterns that could differentiate most (7 out of 8) cases of intestinal T-cell LSA from either normal intestine or T-cell–rich lymphoplasmacytic IBD. In general, intestinal T-cell LSA-associated microRNAs included upregulation of oncogenic microRNAs and downregulation of tumor-suppressive microRNAs. These preliminary findings derived from a small number of intestinal samples suggest that microRNA expression patterns could serve as an adjuvant molecular test to aid in the discrimination of intestinal T-cell LSA from other nonneoplastic intestinal T-cell pathologies.
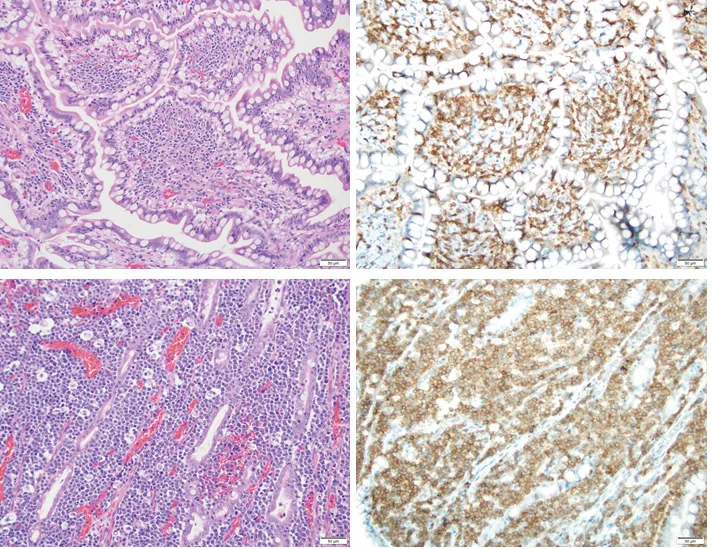
Comparative histology (H&E) and immunophenotype (CD3) of IBD and T-cell LSA affecting the ileum of 2 different dogs. Lymphoplasmacytic IBD and intestinal T-cell LSA can share overlapping histologic findings that can make it difficult to distinguish inflammatory versus neoplastic origin. Differential criteria used to distinguish IBD and LSA include degree of epitheliotropism (LSA > IBD), degree of heterogeneity of inflammatory infiltrate, and cellular interactions with adjacent tissues (expansive nature [IBD] vs infiltrative/effacement nature [T-cell LSA]). With CD3+ lymphocytes, IBD lesions are expansile but not infiltrative, with few epitheliotropic lymphocytes. In comparison, LSA lesions have a high degree of epitheliotropism and monomorphic effacement of underlying enterocytes. Image courtesy of Jonathan Samuelson, University of Illinois at Urbana–Champaign
… The Takeaways
Key pearls to put into practice:
Intestinal pathologies involving T cells can be difficult to clinically and phenotypically distinguish from one another without more advanced molecular diagnostics.
MicroRNAs can regulate cell biologic behavior by altering the expressions of proteins involved in diverse signaling cascades (tumor suppressor and oncogenic pathways).
Select microRNA expression panels can be useful in distinguishing intestinal T-cell LSA from nonneoplastic pathologies such as IBD, and microRNA profiling could be considered a complementary diagnostic test.