Drugs Used for Medical Management of Mast Cell Tumors
Brooke Britton, DVM, DACVIM (Oncology), Schwarzman Animal Medical Center, New York, New York
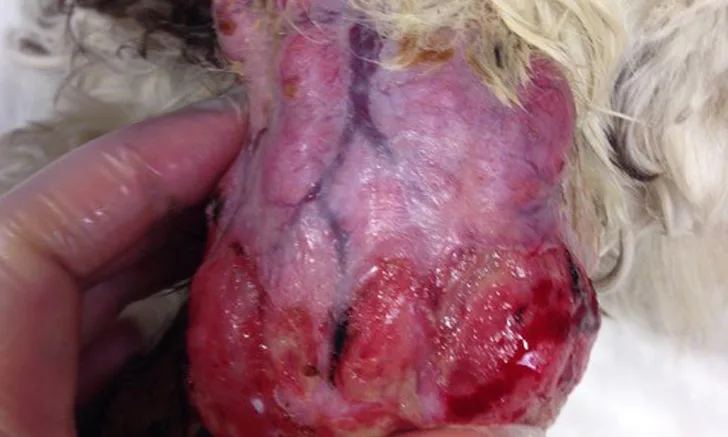
Mast cell tumors (MCTs) are common skin and subcutaneous tumors in dogs and cats that can also less commonly occur as primary tumors within viscera. Degranulation of neoplastic mast cells (randomly or with tumor manipulation) can result in release of histamine, heparin, cytokines, and other vasoactive amines from tumor cell granules,1-3 the sequelae of which may include local tissue reactions (eg, erythema and wheal formation [ie, Darier sign], bruising, edema, tumor surface ulceration) and systemic adverse effects (eg, vomiting, diarrhea, melena or hematochezia, coagulopathy, gastric ulceration, arrhythmias, respiratory distress secondary to effusion, peripheral edema, hypotension, fever).3-6
Therapeutic approaches should focus on reducing the risk for severe or life-threatening complications secondary to MCT degranulation.
The following drugs are typically used in patients with clinical signs likely due to MCTs, prior to surgical manipulation or tumor sampling (eg, via fine-needle aspiration), or when gross disease is present, as in situ degranulation is an ongoing risk.
H1 Antagonists/Antihistamine Agents
Diphenhydramine
Diphenhydramine is a first-generation, H1-receptor antagonist and anticholinergic that blocks certain effects of histamine on MCT degranulation.4,6
Dosage (Dogs, Cats)
2-4 mg/kg PO every 8 to 12 hours6,7
0.5-2 mg/kg SC, IM, or IV once (typical); can also be given every 8 to 12 hours6-8
Key Points
Initial doses should be at the low end of the dose range and escalated over time, particularly in cats, in which this medication can rarely cause paradoxical excitability, and large-breed dogs.6,7
A study of isoflurane-anesthetized dogs undergoing MCT excision found no clear clinical cardiorespiratory benefits of diphenhydramine compared with placebo,8 but antihistaminergic effects can limit vomiting (dogs), pruritus, edema, and wheal formation secondary to degranulation.4,6
Oral bioavailability in dogs is lower than with injectable routes9; injectable diphenhydramine should therefore ideally be used when there is concern for acute degranulation (eg, during tumor sampling via fine-needle aspiration or biopsy).
Oral bioavailability in dogs is estimated to be ≈2% to 8%, with a terminal half-life of 5 hours.9
Oral bioavailability in cats is not well characterized.
Although relevance in veterinary patients is unknown, local tissue necrosis has been reported in humans receiving diphenhydramine SC; caution is therefore advised with SC injection in dogs and cats.10
Adverse effects may include sedation, lethargy, constipation, and urinary retention; vomiting, diarrhea, and anorexia have also been reported.7,11
Caution is advised in patients with glaucoma, prostatic hypertrophy, gastric or urinary obstruction, hypertension, hyperthyroidism, cardiovascular disease, seizure disorders, or asthma.7,11
Cyproheptadine
Cyproheptadine is a first-generation, H1-receptor blocker with anticholinergic and antiserotonergic effects.12
Dosage
Dogs: 0.2-2 mg/kg PO every 12 hours13
Cats: 1-4 mg/cat PO every 12 to 24 hours13
Key Points
Can be used for appetite stimulation in patients with GI upset and inappetence secondary to MCT6,14
Serotonin may be the predominant biogenic amine in feline mast cell granules; cyproheptadine may therefore mitigate effects of degranulation in cats.15,16 Immunohistochemical staining for serotonin in feline MCTs was highest in GI MCTs in one study, suggesting cyproheptadine may be particularly effective in cats with visceral mast cell disease.15,16
Adverse effects may include sedation, dry mucous membranes, urinary retention, decreased GI motility, tachycardia, and paradoxical excitement (cats); seizures can occur with overdose.12,13
Loratadine
Loratadine is a long-acting, nonsedating, second-generation antihistamine that blocks peripheral H1 receptors. This drug is not an alpha-1 antagonist and does not exhibit significant antimuscarinic activity.17,18
Dosage
Dogs: 0.25-1.1 mg/kg PO every 24 hours or divided and given every 12 hours17
Small dogs (<13.2 lb [6 kg]): 5 mg/dog PO every 24 hours17
Medium dogs (13.2-44.1 lb [6-20 kg]): 10 mg/dog PO every 24 hours17
Large dogs (>44.1 lb [20 kg]): 15 mg/dog PO every 12 hours17
Cats:
0.5 mg/kg PO every 24 hours or 2.5-5 mg/cat PO every 24 hours17
Key Points
Dosages are mostly anecdotal. There is no documented efficacy in vivo for this condition; however, in vitro studies have confirmed suppression of immunoglobulin E-dependent histamine release and decreased proliferation in canine mast cell lines exposed to this drug, suggesting a potential therapeutic role.18,19
Adverse effects in humans may include CNS effects (eg, sedation, lethargy, paradoxical excitement), vomiting, tachycardia, dry mouth, or decreased tear production; effects are not well-documented in veterinary medicine.18,19
Caution should be used in patients with keratoconjunctivitis sicca; lower doses should be considered in patients with hepatic or renal impairment.18,19
Chlorpheniramine
Chlorpheniramine is a first-generation antihistamine that competitively inhibits histamine H1 receptors and has anticholinergic, sedative, antispasmodic, local anesthetic, bronchodilator, and antiemetic effects.20
Dosage
Dogs: 0.2-0.5 mg/kg PO every 8 to 12 hours20
Cats: 1-4 mg/cat PO every 8 to 12 hours20
Key Points
May be useful in dampening the effects of histamine release during manipulation or surgical excision of MCTs and in the treatment of MCT degranulation-induced pruritus, particularly in cats21-23
Potential adverse effects include CNS depression (eg, lethargy, sedation), GI upset (eg, anorexia, vomiting, diarrhea, constipation), and anticholinergic effects (eg, dry mouth, urinary or fecal retention).20-23
These adverse effects are common in humans, but incidence in dogs and cats is likely lower.
Paradoxical excitement is possible in cats.
Caution should be used in patients with glaucoma, prostatic hypertrophy, gastric or urinary obstruction, hypertension, hyperthyroidism, cardiovascular disease, renal dysfunction, or asthma.20,22-24
Palatability can be a concern in cats. Tablets can be administered in gelatin capsules, or compounding may be considered, to increase acceptance.22
H2 Antagonists
Cimetidine
Cimetidine competitively inhibits histamine at the H2 receptors on parietal cells, thereby reducing basal gastric acid secretion and gastric acid secretion when stimulated with food or histamine in dogs and cats.25 Cimetidine also has weak antiandrogenic activity and immunomodulatory effects and may reverse regulatory T-cell–mediated immune suppression.26
Dosage (Dogs, Cats)
5-10 mg/kg PO every 6 to 8 hours27
Key Points
Hyperacidity can promote gastroduodenal ulceration and is more likely to occur in dogs and cats with mast cell disease.28,29 Cimetidine and other H2 antagonists can reduce stomach acid levels and treat or prevent stomach ulcers.28,29
Oral bioavailability in dogs is high (≈75%-95%) but can be significantly reduced by food.30
Adverse effects are rare in dogs and cats.
Headache, confusion, increased liver enzymes, pancreatitis, gynecomastia, myalgia (rare), arthralgia, agranulocytosis, arrhythmia, and pain at the injection site with IM administration have been reported in humans.27
Caution should be used in patients with impaired renal or hepatic function.30,31
Affects hepatic metabolism of several drugs and may reduce excretion of drugs with a high first-pass effect, resulting in increased toxicity.
Thorough medical history should be obtained prior to starting therapy to avoid significant drug interactions.25,30,31
May cause small, reversible increases in plasma creatinine concentrations early in the course of therapy25,30
Famotidine
Famotidine competitively inhibits histamine at the H2 receptors of parietal cells, thereby reducing gastric acid production in dogs and cats.32-34 Famotidine has a longer duration of action than cimetidine and fewer reported drug interactions.32-34
Dosage (Dogs, Cats)
1 mg/kg PO, SC, or IV (rounded to the nearest 5 mg) every 12 to 24 hours35
Hospitalized dogs: loading dose of 1 mg/kg slow IV once, then 8 mg/kg/day IV CRI35
Key Points
Prolonged administration can result in decreased effect on intragastric pH in cats and dogs; proton pump inhibitors (PPIs) are therefore preferred for long-term administration (>14 consecutive days) to control stomach acid production and prevent ulcers.32-34,36
Adverse effects are rare. IV administration has anecdotally been reported to cause intravascular hemolysis in cats; however, hemolysis was not noted in 56 hospitalized cats given famotidine IV over 5 minutes.37
Caution should be used in patients with severe cardiac disease, as famotidine has arrhythmogenic properties (ie, bradycardia with rapid IV infusion).34
Ranitidine
Ranitidine competitively inhibits histamine at the H2 receptors on parietal cells, thereby reducing gastric acid output in dogs and cats.38-41 Ranitidine may stimulate GI (particularly gastric) motility through inhibition of acetylcholinesterase and increase lower esophageal sphincter pressure, reducing gastroesophageal reflux.38-41 Ranitidine for humans is no longer available in the United States, Canada, the United Kingdom, Australia, New Zealand, Hong Kong, or countries in the European Union due to unacceptable levels of N-nitrosodimethylamine contamination.42
Dosage
Dogs: 1-2 mg/kg PO, SC, IM, or slow IV (maximum 25 mg/minute) every 8 to 12 hours43
Cats: 2.5 mg/kg slow IV every 12 hours or 3.5 mg/kg PO every 12 hours43
Key Points
Treats and prevents gastric and duodenal ulcers and esophageal reflux secondary to MCTs in dogs and cats and may be useful for chronic therapy in patients in a persistently hypersecretory state (eg, systemic mastocytosis)38-41
Three to 13 times more potent than cimetidine; minimal impact on hepatic metabolism of other drugs, making ranitidine safer in patients with liver disease38-41
Adverse effects are rare but may include vomiting with IV boluses, pain at the injection site after IM administration, agranulocytosis, and cardiac arrythmia (with rapid IV administration).38-41
May cause a false-positive protein reading on urinalysis when using blood and urinalysis test strips43
Proton Pump Inhibitors
Omeprazole is the most commonly used and practical PPI for outpatient treatment of dogs and cats with MCTs. Extra-label use of pantoprazole (0.7-1 mg/kg IV over 15 minutes every 12-24 hours) or esomeprazole (0.5-1 mg/kg slow IV every 12 hours in dogs) can be considered for in-clinic treatment in both cats and dogs; however, validated data regarding pharmacokinetics, adverse effects, and ideal dosages are limited.40,44 Further detail regarding pantoprazole and esomeprazole have thus not been included in this article.
Omeprazole
Omeprazole is a substituted benzimidazole PPI that binds irreversibly at the secretory surface of parietal cells in acidic environments to inhibit >90% of hydrogen ion transport into the stomach.40,41,45
Dosage
Dogs: 1-2 mg/kg PO every 12 to 24 hours46
Cats: 1.1-1.3 mg/kg PO every 12 hours46
Key Points
Prevents and treats gastric ulcers and erosions in dogs and cats
Gastric acid reduction will not be seen for ≈3 to 5 days after therapy is initiated; therefore, concurrent use with an H1 antagonist during initial treatment is warranted in patients at high risk for gastric ulcers (eg, with systemic mastocytosis or large tumor burden) or with known or suspected ulceration.32,40,41,45
Enteric-coated tablets or capsules should not be broken or crushed, as omeprazole is rapidly degraded by stomach acid; omeprazole formulation ensures maximum absorption in the proximal duodenum after gastric emptying.32,45-47
Actively metabolized in the liver to multiple different metabolites and excreted principally in the urine
Dose adjustments in patients with severe renal or hepatic dysfunction should be considered.32,40,41,45
Potential adverse effects include GI upset (eg, nausea, vomiting, anorexia, diarrhea, flatulence), proteinuria, and CNS disturbances.40,41,45
Appears to be safe in dogs for ≤4 weeks.40,45 Long-term therapy in humans has been associated with bone fractures, hypomagnesemia, fundic polyps, and acute interstitial nephritis.47 Risks of chronic use in dogs and cats have not been well-defined.
GI Mucosal Protectants
Sucralfate
Sucralfate’s exact mechanism of action is unknown but is thought to act locally on ulcerated gastric mucosa by reacting with hydrochloric acid to form a paste-like substance that binds to proteinaceous exudates at ulcer sites.48 Sucralfate may also have cytoprotective effects, putatively through stimulation of prostaglandin E2 and I2.49,50
Dosage
Dogs:
Small dogs (<33 lb [15 kg]) and toy breeds: 250-500 mg PO as a slurry mixed with water every 6 to 12 hours51
Large dogs (>33 lb [15 kg]): 1 g PO as a slurry mixed with water every 6 to 12 hours51
Cats: 250-500 mg PO as a slurry mixed with water every 6 to 12 hours51
Key Points
Used to treat known or suspected active gastroduodenal ulcers in dogs and cats
May bind or decrease bioavailability of several drugs and therefore should be administered at least 2 hours after other medications52-54
Concurrent use with PPIs or H2 antagonists does not appear to have improved outcomes versus sucralfate alone.50
Adverse effects may include constipation (dogs) or vomiting (cats).50,52-55
Prostaglandin Analogues
Misoprostol
Misoprostol is a synthetic prostaglandin E1 analogue that acts directly on parietal cells to inhibit basal and nocturnal gastric acid secretion, as well as acid secretion stimulated by histamine.52,54-56 Misoprostol also has a cytoprotective effect on gastric mucosa and enhances mucosal healing in response to acid-related injury.52,54-56
Dosage
Dogs: 2-5 µg/kg PO every 8 to 12 hours57
Cats: 5 µg/kg PO every 8 hours57
Key Points
Used mainly for prevention of GI ulcers in dogs and cats; may not be as effective in treatment of active ulcers and appears ineffective in treatment of ulceration due to glucocorticoid-induced mucosal injury50,52,54,55
Adverse effects primarily include GI upset (eg, diarrhea, vomiting, abdominal pain, flatulence).50,52,54,55
Misoprostol is an abortifacient medication that is therefore contraindicated in pregnant dogs and cats and should not be handled by women who are pregnant or attempting to become pregnant.50,52,54,55
Glucocorticoids
Prednisone/Prednisolone
Prednisone and prednisolone are intermediate-acting synthetic glucocorticoids that inhibit MCT proliferation, induce apoptosis in vitro, and reduce peritumoral edema and inflammation.6,58
Dosage
Dogs: 0.5-2 mg/kg PO every 24 hours59
Cats (prednisolone): 1-2 mg/kg PO every 24 hours59
Key Points
Effective in treatment of many secondary effects of MCT degranulation and improvement of patient appetite and energy level, as well as cytoreduction of MCTs in dogs and cats60-64
Although there are established dose ranges for dogs and cats, clinician discretion regarding what dose to administer in these ranges should be used when treating patients with MCT and should depend on the patient’s unique clinical presentation. Administration typically begins with a midrange to higher dose tapered to the lowest effective dose; however, some patients may require a consistent higher dose over a longer period or only a transient tapering course that can be discontinued.
Typically provides short-term benefits (weeks to months) when used as single-agent therapy for MCTs, particularly for late-stage mast cell disease and systemic mastocytosis65
Prednisone is a prodrug that requires hepatic activation and has lower bioavailability in cats; prednisolone is therefore preferred in cats with MCTs and dogs with severe hepatic disease.59,64
Common adverse effects include polyuria, polydipsia, polyphagia, weight gain, panting (dogs), and elevated liver enzymes (dogs). GI upset, hyperlipidemia, hypercoagulability, muscle wasting, behavioral changes, and hyperglycemia can also occur.60-64 Cats appear to be more susceptible to hyperglycemia.64
Caution should be used, or this drug avoided, in patients with hyperadrenocorticism, congestive heart failure, hypertension, diabetes, bacterial or fungal infection, or active gastroduodenal ulceration.64
Triamcinolone
Triamcinolone is an intermediate-acting glucocorticoid ≈4 to 10 times more potent than hydrocortisone.65
Dosage (Dogs, Cats)
1.2-1.8 mg intralesional injection (not to exceed 0.6 mg at any one site or 6 mg total dose)66
Key Points
Intralesional injection has been reported in dogs for treatment of isolated local tumors; overall response rate was 67% when used alone or in combination with other therapies, including oral glucocorticoids, radiation, chemotherapy, and/or a tyrosine kinase inhibitor.67
Typically used for treatment of smaller (<1 cm), isolated lesions when surgery cannot be performed or is declined
There is no long-term benefit in patients with larger tumors or late-stage (metastatic) disease.67
An ideal protocol for cats has not been published.
Adverse effects are uncommon but may include mild hemorrhage at the injection site and GI upset.66,67
Novel Small Molecule Agents
Tigilanol Tiglate
Tigilanol tiglate is a novel small molecule agent that destroys MCTs in dogs by direct oncolysis of tumor cells through mitochondrial disruption, by provoking an acute inflammatory response that restricts tumor blood and oxygen supply and activates innate immune responses, and by increased permeability of tumor vasculature through activation of protein kinase C.68
Dosage (Dogs)
0.5 mL/cm3 of tumor volume per single MCT intralesional injection (minimum dose, 0.1 mL; not to exceed maximum tumor volume of 10 cm3 or total dose of 5 mL/dog or 0.25 mL/kg)68,69
Intralesional injection may be repeated after 30 days if an incomplete response is obtained with initial treatment.68,70
Key Points
Approved for intralesional injection in dogs with nonmetastatic cutaneous (anywhere on the body) or nonmetastatic subcutaneous (at or below the elbow or hock) MCTs; should not be used in cats67-69
Injection should be directly into the tumor only (never at the tumor periphery or into adjacent tissues) because of the risk for severe tissue necrosis.70-72
Concurrent use with a corticosteroid, H1 antagonist, and H2 antagonist is needed because of the risk for severe MCT degranulation postinjection.70-72
Corticosteroid (prednisone/prednisolone, 0.5 mg/kg PO every 12 hours for 7 days, then every 24 hours for 3 days; total of 10 days) should be started 2 days prior to injection with tigilanol tiglate and continued for 7 days postinjection.
H1-antagonist (diphenhydramine, 2 mg/kg PO every 12 hours) and H2-antagonist (famotidine, 0.5 mg/kg PO every 12 hours) therapy should be started the day of injection with tigilanol tiglate and continued for 7 days postinjection (total of 8 days).
Failure to administer concomitant medications as recommended can result in death.68-72
Common adverse effects are swelling, bruising, and pain at the injection site; lameness in the treated limb; GI upset (eg, vomiting, diarrhea, anorexia); hypoalbuminemia; and significant open wound formation at the injection site.68-72
Extreme care should be used to avoid accidental self-injection when administering tigilanol tiglate, as there is potential for severe wound formation.68,70,72