Differentiating Canine Urinary Tract Infections
Gregory F. Grauer, DVM, MS, DACVIM (SAIM), Kansas State University
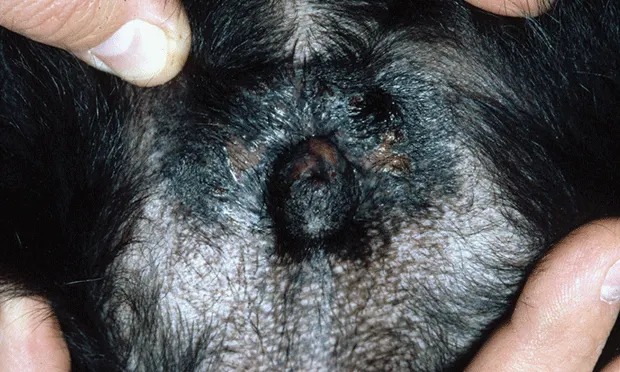
Radiograph of the vulvar region demonstrating recessed vulva and perivulvar dermatitis.
Georgia, an 8-year-old spayed black Labrador retriever, was presented with urinary incontinence of several months’ duration.
History
Georgia’s incontinence, most pronounced when she was sleeping or lying down, had been present for several months and preceded more recent signs of lower urinary tract inflammation (ie, pollakiuria, dysuria/stranguria, breaking normal house-training behavior).
Examination
Georgia was slightly overweight and had excessive perivulvar skinfolds with some perivulvar inflammation and evidence of licking and pigmentation change (Figure 1). Physical examination, including rectal examination and palpation of the urinary bladder, was unremarkable.
Initial Laboratory Evaluation
CBC and biochemistry profile were within normal limits. Urinalysis obtained by cystocentesis revealed a cloudy appearance with pH of 7.5, urine specific gravity of 1.037, 2+ proteinuria, 25 to 30 RBCs/hpf, 10 to 15 WBCs/hpf, 25 struvite crystals/hpf, and gram-negative rods. Urine culture yielded Escherichia coli (>1000 cfu/mL) sensitive to amoxicillin–clavulanic acid.
Plain film radiographs of the abdomen followed by a double-contrast cystogram demonstrated a pelvic bladder (Figure 2).

Double-contrast cystogram demonstrating the pelvic bladder.
Simple vs Complicated Urinary Tract Infections
Simple or uncomplicated urinary tract infections (UTIs) lack structural or functional abnormalities in the host’s defense mechanisms. This form of infection is easiest to treat and usually clears soon after appropriate antibiotic treatment. Simple, uncomplicated UTIs are the most common type to occur in female dogs.
Complicated UTIs are associated with 1 or more defects in the host’s defense mechanisms: for example, interference with normal micturition, anatomic defects, damage to mucosal barriers, or alterations in urine volume or composition. Health of host defense mechanisms appears to be most important in influencing the pathogenesis of UTIs. Although antibiotic treatment is the cornerstone of UTI management, status of host defense mechanisms is thought to be the most important determinant of long-term treatment outcome. Antibiotic treatment should control the pathogenic bacterial growth for a period sufficient to allow host defense mechanisms to be corrected and prevent colonization of the urinary tract without further antibiotic administration.
Diagnosis
Georgia had a UTI caused or complicated by probable urethral sphincter mechanism incompetence (USMI), abnormal vulvar anatomy, and subsequent perivulvar inflammation. The pelvic bladder location results in a shortened urethra, which has been associated with USMI. Decreased urethral sphincter tone allows bacteria to more easily ascend to the bladder. Abnormal vulvar anatomy and USMI resulted in a moist perivulvar dermatitis and increased the number of pathogenic bacteria at the vulvar opening. Excessive perivulvar skinfolds likely occurred secondary to weight gain.
Treatment
Georgia was treated with amoxicillin–clavulanic acid at 13.75 mg/kg PO q12h for 4 weeks, indefinite administration of phenylpropanolamine at 1.5 mg/kg PO q12h for sphincter incompetence, local treatment (astringents, hot-packing, topical antibiotics), and an Elizabethan collar to prevent licking of the perivulvar dermatitis.
Follow-up
The owner reported that the vulva appeared improved and the patient rarely leaked urine and showed none of the previous signs of lower urinary tract inflammation. Urine sediment was inactive. Urine culture 10 days after antibiotic withdrawal showed no growth. Approximately 2 months after antibiotic treatment was discontinued, Georgia again showed signs of lower urinary tract inflammation (ie, pollakiuria, breaking house training). Urine culture obtained by cystocentesis again yielded E coli but with a markedly different sensitivity profile (sensitive to fluoroquinolones).
Recurrent UTIs
Relapses are infections caused by the same species of bacteria, usually within several days of treatment cessation. With a relapsed UTI, previous antibacterial treatment failed to eliminate infection. Relapses may be caused by improper antibiotic or dose, emergence of drug-resistant pathogens, or failure to eliminate predisposing causes that alter normal host defense mechanisms and allow bacteria to persist (eg, viable bacteria sequestered within struvite uroliths).
In reinfections, previous antibacterial treatment can clear the first infection and the urinary tract subsequently becomes infected with another bacteria. The time between reinfections is usually greater than the time between relapses. Reinfections often indicate failure to eliminate predisposing causes that alter normal host defense mechanisms.
Because of the length of time between the UTIs and markedly different sensitivity profile, Georgia’s recurrent UTI is most likely a reinfection with a different uropathogenic E coli. Even though the phenylpropanolamine and local treatment of the perivulvar dermatitis helped improve host defense mechanisms, abnormalities were still present (USMI, abnormal vulvar anatomy). Subclinical urinary incontinence associated with USMI may persist despite phenylpropanolamine treatment. In addition, episioplasty may be considered if the perivulvar dermatitis persists or recurs.
Ancillary therapies designed to prevent recurrent UTIs are considered in cases where breaches in host defenses are present but not correctable or in which an underlying cause for reinfection is not identified. Ancillary therapies include urinary antiseptics and acidifiers, CE (see Cranberry Extract & E coli), and prophylactic long-term, low-dose antibiotic treatment. Of note, these ancillary therapies have not been proven effective in controlled, prospective clinical trials.
Outcome
Once Georgia’s recurrent UTI had been effectively treated (follow-up urine cultures had no growth), long-term CE treatment was initiated to help support her compromised host defense mechanisms. Recheck urinalyses were performed quarterly. Long-term prophylactic antibiotic treatment was not used because of the potential for creating an antimicrobial-resistant UTI.
Cranberry Extract & E coli
Proanthocyanidins, specifically A-type isoforms found in cranberry extract (CE), can potentially inhibit E coli attachment by blocking interaction of bacterial P-fimbriae with the surface of uroepithelial cells. Canine studies evaluating the efficacy of CEs are limited to in vitro data. One study demonstrated that E coli in urine from dogs receiving oral CE (Crananidin, nutramaxlabs.com) had decreased ability to agglutinate to human RBCs. Similarly, a second study demonstrated that E coli in urine from dogs receiving oral CE (Paxon, vetoquinolusa.com) had decreased ability to adhere to Madin-Darby canine kidney cells.
Based on these studies, oral administration of CE may help reduce E coli reinfections in patients with compromised host defense mechanisms.1,2
Text at a Glance:
Complicated & Recurrent UTIs
Long-term antibiotic treatment (4–6 weeks) based on culture and sensitivity
Thorough patient assessment for compromised host defense mechanisms
Chemistry profile and follow-up testing to rule out systemic immunocompromise (eg, chronic kidney disease, diabetes mellitus, hyperadrenocorticism)
Imaging to rule out local anatomic abnormalities
Correction/control of compromised host defense mechanisms, if possible
Prophylactic preventive measures if host defense mechanisms cannot be corrected (only after appropriate antibiotic treatment has sterilized the urinary tract)
Urinary antiseptics/acidifiers
CE
Long-term, low-dose prophylactic antibiotic treatment
CE = cranberry extract, USMI = urethral sphincter mechanism incompetence, UTI = urinary tract infection