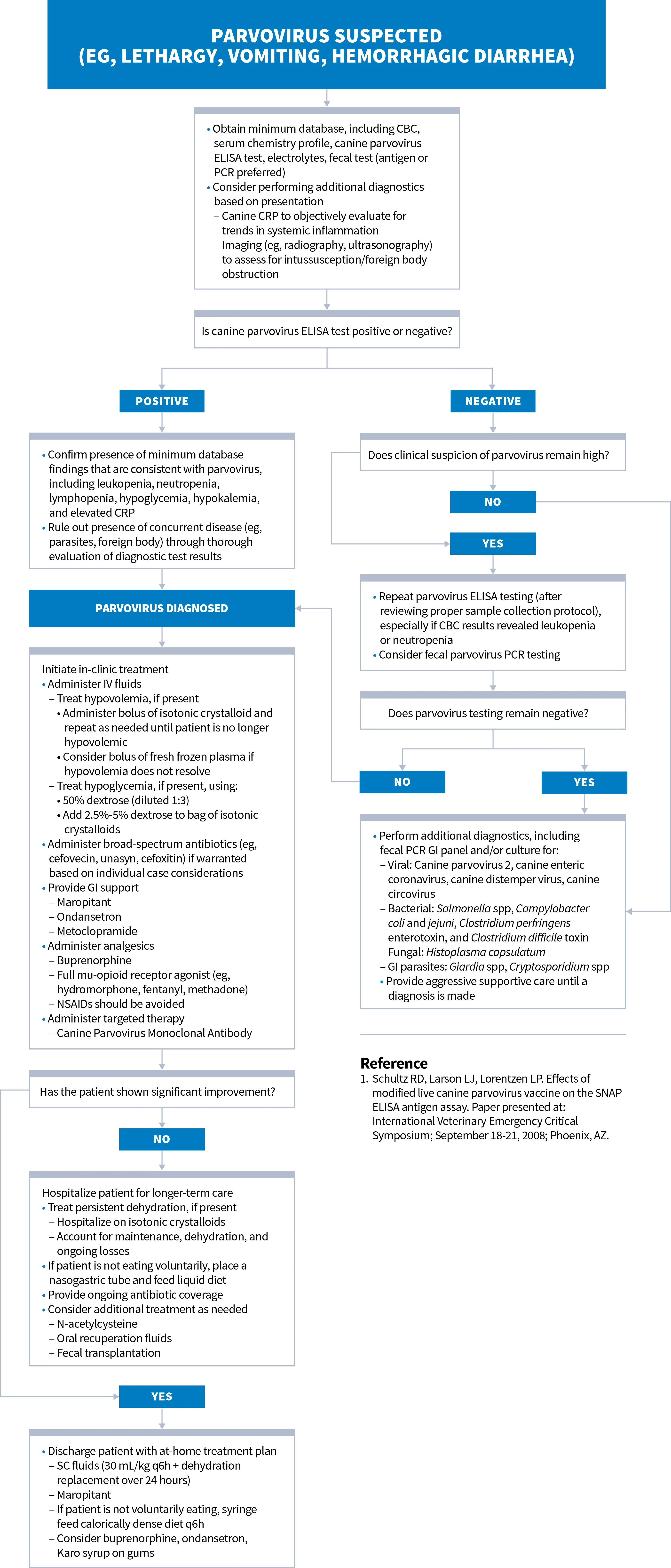
Diagnosis & Treatment of Canine Parvovirus
Kristin Zersen, DVM, DACVECC
Fred Metzger, DVM, MRCVS, DABVP
Sponsored by Elanco
Canine Parvovirus Monoclonal Antibody (CPMA)
CPMA is conditionally approved by the USDA for treatment of canine parvovirus in dogs 8 weeks of age and older.
CPMA is administered IV at a dose of 0.2 mL/kg (0.2 mL/2.2 lb) given at the time of diagnosis.
CPMA is delivered in a unique freezer package that allows for proper storage until the time of use.
In a treatment efficacy study, dogs receiving CPMA experienced faster resolution of vomiting, inappetence, and lethargy, and no dogs in the study died of CPV.
Clinical Considerations for Parvovirus
Special consideration must be given to evaluate for concurrent disease in cases of parvovirus, especially GI parasitism.
Salmonella and sepsis should be strongly considered in ELISA-negative patients with leukopenia.
Fecal PCR testing should be considered for patients with negative ELISA results. Monitoring serial CBC and CRP values may be useful in informing the prognosis and guiding timing of patient discharge.
In a study, the virus was not detected in the stool of dogs that had recently received the parvovirus vaccine, suggesting false-positive ELISA tests are uncommon.1
PM-US-23-1583
