Cytology Challenge: Unusual Sediment Findings

The checklist for identification of common urine sediment findings includes cells (eg, epithelial cells, erythrocytes, leukocytes), crystals (eg, struvite, calcium oxalate, bilirubin, ammonium biurate), and microorganisms (typically bacteria). Some practitioners, however, may encounter unusual findings that hold great diagnostic value. In the cases that follow, unusual urine sediment findings yielded interesting, if not diagnostically critical, information.
CASE 1
Purulent Vaginal Discharge and Caudal Abdominal Pain
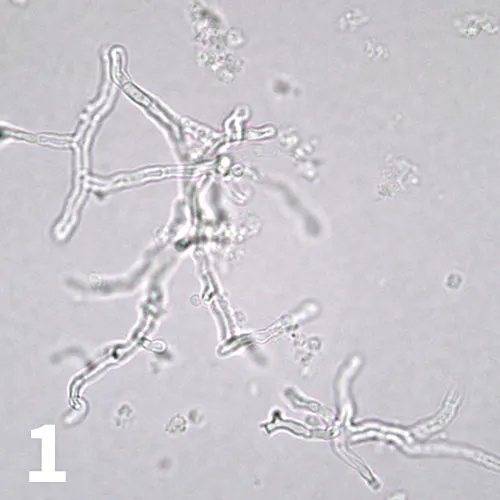
Unstained urine wet mount, 50× objective. Branching structures with parallel walls and perpendicular septa are present. Courtesy of Diagnostic Cytology and Hematology of the Dog and Cat, Valenciano AC, Cowell RL (eds).
A 5-year-old spayed beagle was presented with purulent vaginal discharge and caudal abdominal pain. Minimum database testing with urinalysis on a specimen obtained by cystocentesis showed an inflammatory leukogram, hyperglobulinemia, renal azotemia (elevated BUN and creatinine with concurrent isosthenuria), moderate proteinuria, and marked pyuria with gross hematuria. Cytologic examination of a urine sediment wet-mount specimen is shown (Figure 1).
Related Article: Image Gallery: Urinalysis in Small Animals
ASK YOURSELF
What are the branching structures?
What additional diagnostic tests may be useful to confirm and better characterize this patient’s disease?
Diagnosis
Systemic Aspergillosis
Interpretation & Discussion
Ultrasonography showed cystic areas in the kidneys; fine-needle aspiration and cytology were pursued. In a Wright-Giemsa–stained specimen (Figure 2), the branching hyphae with parallel cell walls were seen in the midst of dense nuclear debris and markedly karyolytic, degenerate neutrophils. Fungal hyphae showed variable staining cytologically. The hyphae may be nonstaining, as in this case, or they may stain bright blue with a thin, colorless cell wall.
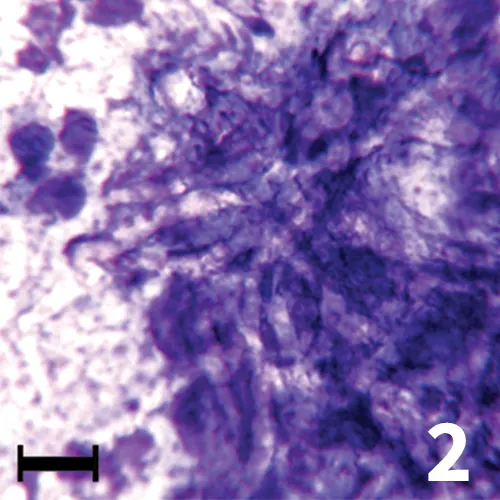
Wright-Giemsa–stained renal aspirate, 50× objective, bar = 10 µm. Large, non-staining, branched fungal hyphae are seen against a background of nuclear debris.
To better visualize the fungal hyphae, a specialized silver stain was also used; the hyphae appear black with this special stain, and the septae are better visualized (Figure 3). This special stain can be performed on routine cytologic samples by reference laboratories.
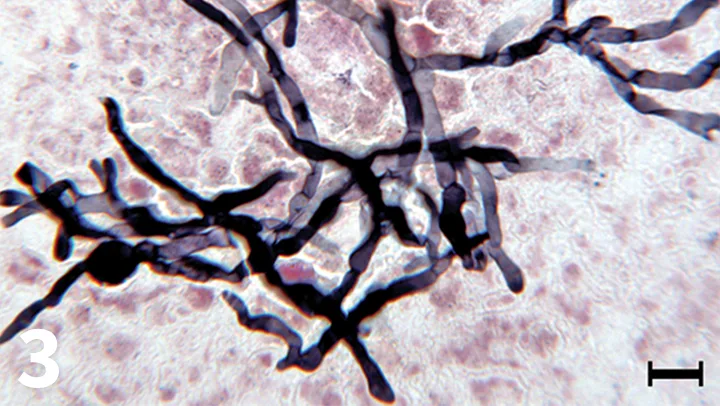
Gomori methenamine silver staining of a renal aspirate, 50× objective, bar = 10 µm. The branching, septate fungal hyphae are black in the silver-stained preparation.
Culture of urine showed Aspergillus species, a ubiquitous saprophytic fungus that may cause opportunistic infections. In 1 study, urinalysis findings in dogs with systemic aspergillosis included hematuria, pyuria, and fungal hyphae in the sediment, in decreasing order of frequency. Female dogs and German shepherd dogs appear to be overrepresented.1
DID YOU ANSWER?
Fungal hyphae. In this case, the diagnosis was Aspergillus spp infection.
Fungal culture or serologic testing.
CASE 2
Clinically Normal Patient with Chronic Antibiotic Administration
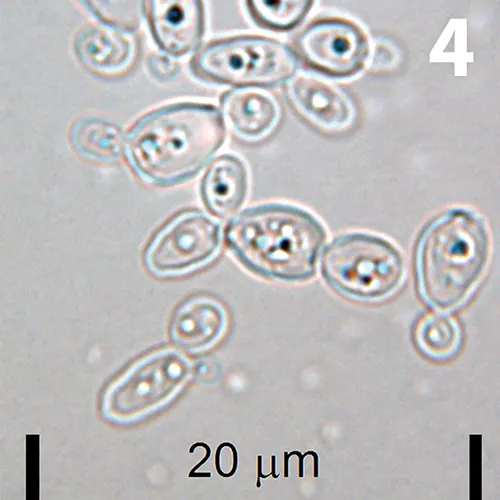
Unstained urine wet mount, 100× objective. A few clusters of oval organisms are shown.
An adult spayed female domestic shorthaired cat was undergoing routine annual examination with laboratory evaluation. The patient had received a potentiated β-lactam at the labeled dose intermittently for chronic upper respiratory infection and had recently finished a 7-day course. A photomicrograph of a wet-mount preparation of urine sediment obtained on cystocentesis is shown in Figure 4.
ASK YOURSELF
What are the oval structures?
What part of this patient’s history likely contributed to this urine sediment finding?
Diagnosis
Urinary Candidiasis, Likely Secondary to Chronic Antibiotic Administration
Interpretation & Discussion
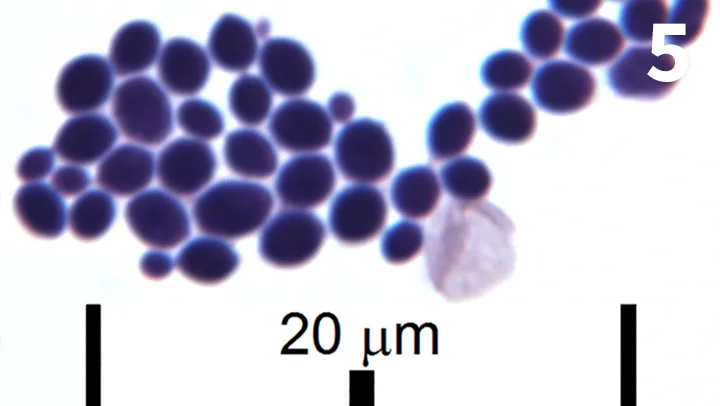
Wright-Giemsa–stained urine dry mount, 100× objective. A cluster of 2- to 3-µm yeast (sometimes budding) is shown along with an erythrocyte near the center of the field.
The patient’s urine contained large numbers of yeast cells, some of which were budding (Figure 5); no other evidence of cystitis was present. This is an uncommon finding. False diagnosis of yeast cystitis is possible if the yeast identified microscopically is actually from wet mount stain that is contaminated by yeast or from the patient’s genitals or skin. These potential sources of contamination are avoided by examination of a fresh urine sample obtained by cystocentesis that is prepared as a plain, unstained wet mount.
Yeast cystitis is typically a secondary pathology. Considerations for primary disease include bacterial cystitis, uroliths, and urinary catheterization, as well as chronic antibiotic use, diabetes mellitus, and other immune-compromising disorders.2
DID YOU ANSWER?
Budding yeast, identified as Candida spp on culture.
The patient’s chronic antibiotic administration likely contributed to the yeast infection.
CASE 3
Partial Urethral Obstruction
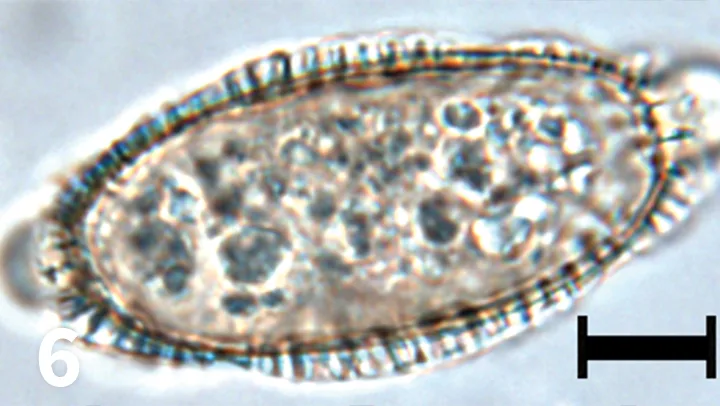
Unstained urine wet mount, 50× objective, bar = 10 µm. A large, oval structure is present.
A 12-year-old indoor-outdoor castrated domestic shorthair cat was presented with stranguria, dysuria, and pollakiuria. Urinalysis with sediment evaluation was performed in-house. Based on the findings, contamination of the urine sample with fecal material was suspected, so the sediment was submitted to a reference laboratory for review (Figure 6).
ASK YOURSELF
What is the structure?
Which species may be affected with this condition?
What is the most common presenting complaint associated with this condition?
Why was the referring veterinarian concerned about fecal contamination of the urine sample?
Diagnosis
Capillariasis
Interpretation & Discussion
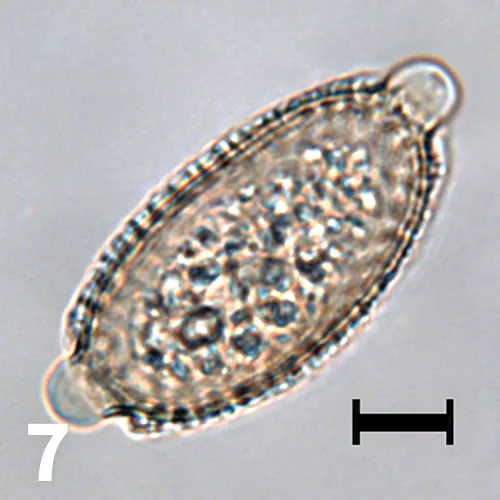
Unstained wet mount, 50× objective, bar = 10 µm. A Capillaria plica ovum is presented to better show the mammillated shell and opercula, which are slightly askew from 180°.
Capillaria plica (Pearsonema plica) is a nematode found in the urinary bladder of cats, dogs, foxes, and other wild canids. The eggs may be confused with the eggs of Trichuris vulpis because of the bipolar opercula. However, T vulpis is an intestinal parasite. The opercula of C plica eggs are usually slightly askew, whereas those of T vulpis tend to be perfectly bipolar.
The shells of C plica eggs have a rougher, mammillated surface (Figure 7), whereas those of T vulpis are smooth. Ingestion of the earthworm intermediate host containing infective larvae is the likely mode of transmission. Although most patients are clinically normal, lower urinary tract signs have been observed in dogs, while inflammation and urethral obstruction have been observed in cats.3,4
DID YOU ANSWER?
Capillaria plica ovum.
Cats, dogs, and foxes.
Patients are usually clinically normal.
Capillaria ova resemble those of Trichuris vulpis, or whipworm.
Related Article: A Closer Look at Urine Casts
CASE 4
Weight Loss and Chronic Cough
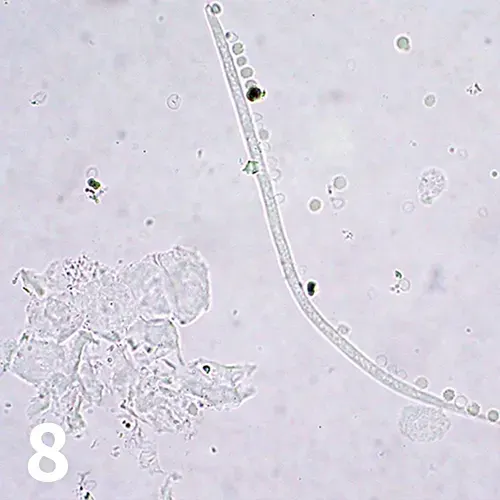
Unstained wet mount, 40× objective. A slender larva with a tapered tail is shown. Hematuria, which was likely iatrogenic, is also observed. Squamous cells are seen at the lower left and right.
A previously stray, middle-aged, crossbreed dog was presented with inability to gain weight and chronic intermittent cough. Initial minimum database evaluation for respiratory and metabolic disease, including urinalysis on a sample obtained by cystocentesis, was performed. A photomicrograph of a wet-mount preparation of urine sediment is shown in Figure 8.
ASK YOURSELF
Could the larvae be related to the patient’s respiratory signs?
Diagnosis
Dirofilaria immitis Infection
Interpretation & Discussion
This was an unusual finding. Larvae are not usually seen in urine. Primary urinary system nematodes in small animals include Capillaria species and Dioctophyme renale, both of which may be diagnosed by finding representative ova in the urine.
Primary urinary system nematodes in small animals include Capillaria species and Dioctophyme renale, both of which may be diagnosed by finding representative ova in the urine.
Larvae of Capillaria species have rarely been reported in feline urine sediment,4 and those of D renale are not usually seen. No ova were found in the urine sediment. However, this patient had a patent Dirofilaria immitis infection diagnosed by occult heartworm ELISA. Microfilariae were also found on blood smear examination. The urine sediment microfilariae were likely contaminants caused by iatrogenic hemorrhage during collection.
DID YOU ANSWER?
Yes. The larva was a contaminant from the iatrogenic hemorrhage in the sample and was consistent with this patient’s diagnosis of D immitis infestation.
CASE 5
Urethral Obstruction
A 5-year-old intact male Australian cattle dog was presented with stranguria with minimal-to-no urine production. Physical examination showed pain on palpation of the caudal abdomen and distention of the bladder.
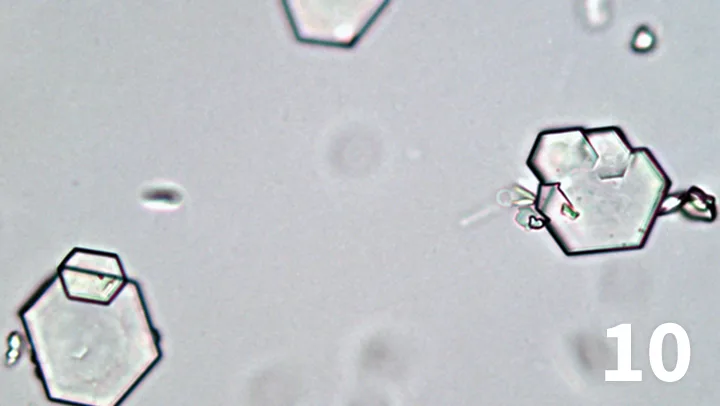
Figure 9. Unstained urine wet mount, 50× objective. Flat, hexagonal, and sometimes irregular-shaped, colorless crystals are shown. They frequently overlay one another. Smaller daughter crystals are sometimes seen budding from a larger crystal.

Unstained urine wet mount, 50× objective. Flat, hexagonal, and sometimes irregular-shaped, colorless crystals are shown. They frequently overlay one another. Smaller daughter crystals are sometimes seen budding from a larger crystal.
Imaging disclosed urinary bladder and penile urethral calculi just proximal to the os penis. Urinalysis was performed and crystals were observed in a wet-mount preparation of the urine sediment (Figure 9).
ASK YOURSELF
What type of crystal is present?
What usually causes the formation of these crystals?
Under what pH conditions are these crystals formed?
Diagnosis
Cystine Urolithiasis
Interpretation & Discussion
Urolith analysis was consistent with the urinalysis findings of cystinuria. Cystine crystalluria and urolithiasis is an uncommon but well-documented condition in dogs. Recent studies have looked at the molecular basis and modes of inheritance of different types of canine cystinuria.5 It is caused by an inherited renal tubular transport defect in which affected individuals cannot reabsorb dibasic amino acids.
Unstained urine wet mount, 50× objective. The cystine crystals are seen superimposed on each other with smaller crystals budding from larger ones.
Although other amino acids (eg, ornithine, lysine, arginine) are also lost in the urine, cystine is the most clinically relevant as its low solubility in acidic or neutral urine leads to the formation of crystals and uroliths along the urinary tract.6
Many breeds, including English bulldogs, dachshunds, Newfoundlands, Australian cattle dogs, Labrador retrievers, other purebreds, and crossbreeds, have been affected. Cystine crystalluria is a risk factor for urolithiasis and should prompt ultrasonographic evaluation for uroliths.
DID YOU ANSWER?
These are cystine crystals (Figure 10), which are flat, hexagonal, and colorless and tend to form stacks.
An inherited renal tubular transport defect is usually the cause.
Acidic or neutral urine favors formation of cystine crystals.
CASE 6
Refractory Seizures
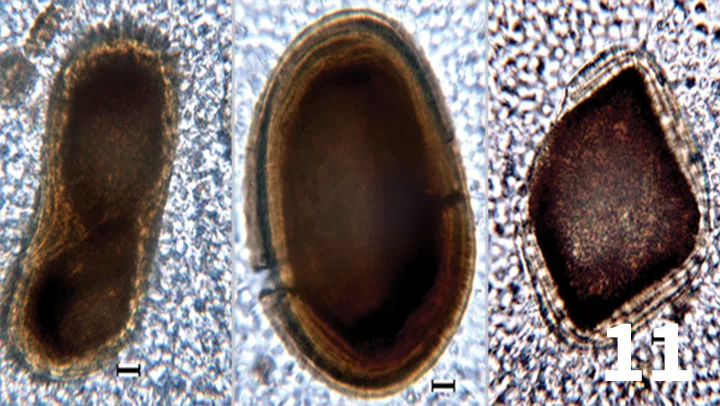
Unstained urine wet mount, 50× objective, bar = 10 µm. Photomicrographs showing crystalline structures that were numerous in the urine sediment. The crystals are dark brown, variably shaped, and exhibit a concentric layering effect. Courtesy of Diagnostic Cytology and Hematology of the Dog and Cat, Valenciano AC, Cowell RL (eds).
A 15-year-old castrated Jack Russell terrier was presented with seizures that were refractory to medical management. During hospitalization, medications included zonisamide, levetiracetam, dexamethasone, acetylcysteine, furosemide, and mannitol. As part of the diagnostic evaluation of neurologic disease, a urinalysis was performed. Photo-micrographs of the urine sediment are shown in Figure 11.
ASK YOURSELF
Are the crystalline structures of a common morphology seen in dogs?
What is the likely composition of the crystal structures?
Diagnosis
Intracranial Mass
Interpretation & Discussion
The patient’s refractory seizures were associated with an intracranial mass. The polypharmacy used to treat this patient was likely the cause of the crystals. A number of drugs have been associated with crystalluria in human and veterinary patients. Anticonvulsant polypharmacy has specifically been studied in the human literature. Patients receiving zonisamide, a sulfa-containing medication, and patients taking multiple medications frequently had crystalluria.7
DID YOU ANSWER?
No, the crystals are unusual.
The crystals are likely formed from precipitated drugs or drug metabolites, given the patient was on multiagent therapy that included anticonvulsant drugs.
Related Article: Urine Crystals in Dogs & Cats
Conclusion
Although the urine sediment findings in this series are not common, they did prove useful in the diagnosis or confirmation of many of the disease processes presented. The series highlights the diagnostic utility of urine sediment evaluation beyond the typical checklist used in most practices and the importance of pursuing unexpected or unusual laboratory findings.
AMY L. WEEDEN, DVM, recently completed her clinical pathology residency at University of Florida. Prior to her residency, Dr. Weeden completed a shelter animal medicine and surgery internship at Colorado State University and was an associate in private small animal practice for 8 years. She is a graduate of Louisiana State University.
HEATHER L. WAMSLEY, DVM, PhD, DACVP (Clinical Pathology), is veterinary clinical pathologist at Antech Diagnostics in Tampa, Florida. Since 2004, Dr. Wamsley has instructed urinalysis and other clinical pathology wet labs at the NAVC Conference, in addition to participating in a hematology masterclass and cytology symposium. A graduate of University of Wisconsin–Madison, she earned a doctorate in molecular biology of infectious diseases from University of Florida.