Conjunctival Samples in Dogs & Cats
Sheryl G. Krohne, DVM, MS, DACVO, Purdue University
Surface diseases that involve the conjunctiva of the eyelids or globe or affect the cornea are the most commonly treated ocular diseases in small animal practice. When it is not possible to establish a definitive diagnosis through clinical presentation alone, cytologic and histopathologic evaluations are useful diagnostic tools. Often overlooked, these evaluations can pinpoint a diagnosis or at least rule out many diseases on the differential list. In addition, accurate and appropriate diagnoses from cytologic and histopathologic ocular tissue samples are often more reliable than culture results, especially for viral diseases. These tests may be underused due to lack of education regarding what information they can provide, lack of training in how to perform the procedures, or a misconception that general anesthesia or sedation is always necessary. Although chemical restraint may occasionally be required, most patients tolerate conjunctival procedures with only topical anesthesia.
Feline Diseases
Feline conjunctival diseases that are most readily diagnosed through cytologic and histopathologic evaluation include conjunctival feline herpesvirus (FHV-1) (Figure A), Chlamydophila species, Mycoplasma species, or other bacterial infections; proliferative (eosinophilic) conjunctivitis, which can be nodular or diffuse (Figure B); keratoconjunctivitis sicca; allergic conjunctivitis; and neoplasia. Because topical and systemic treatments for these diseases are very different and using the wrong treatment (eg, corticosteroids used in the case of herpesvirus) can lead to deep corneal ulceration and possible perforation of the globe, it is important to diagnose the etiology of conjunctivitis correctly.
Canine Diseases
Canine conjunctival diseases that are most readily diagnosed through cytologic and histopathologic evaluation include allergic or follicular conjunctivitis (Staphylococcus [Figure C], pollen and/or dust hypersensitivity); keratoconjunctivitis sicca (KCS); episcleritis (Figure D); foreign body reaction; canine distemper; Thelazia (parasitic) conjunctivitis; conjunctival plasmoma of the third eyelid from pannus; type I hypersensitivity (insect bite [Figure E]); nonneoplastic granulomatous disease (eg, nodular granulomatous episclerokeratitis or collie granuloma); and occasionally neoplasia.

FIGURE A
What You Will Need
One or 2 assistants to restrain the patient and hold the eyelids open
Proparacaine 1% topical ophthalmic drops · 2.5× to 4× magnification head loupe and surgical light illumination · Bishop-Harmon (0.8 mm) or Lester fixation (1 × 2 angled teeth) forceps
Tenotomy or iris scissors
Epinephrine (1/10,000 dilution using stock concentration and saline) or 2.5% phenylephrine ophthalmic solution and cotton swabs to control bleeding from small vessels
10 glass slides and small tube or jar of formalin (for biopsy) · No. 15 scalpel blade, Kimura spatula, and/or small disposable dental brush or cotton swab
Diff-Quik or other Wright-Giemsa type stain; Gram stain for bacteria if you are doing your own staining
Step-by-Step: Conjunctival Biopsy
Step 1
Topical proparacaine (1%) should be placed on the conjunctival area to be biopsied; 1 or 2 drops at a time, spaced 30 seconds apart, 3 or 4 times. Some patients will allow you to place a proparacaine-soaked cotton-tipped applicator on the site, holding it there for 10 seconds after the drops have been placed. Using moist cotton balls, clean enough discharge from the eyelids and conjunctival sac before the procedure to allow for a clear view of the biopsy site.

Step 2
Proper restraint involves controlling the head without squeezing the muzzle too hard. Place one hand behind the back of the head and the other hand around the nose, controlling the body with the elbow. A good light source aimed at the biopsy site is required. Preplace the beam in a comfortable position and have the assistant move the animal's head and eye into the lighted area and keep it there during the biopsy. It is important to use a head loupe for magnification; the recommendation is 2.5× to 4× magnification with a 12- to 24-inch working distance.

Step 3
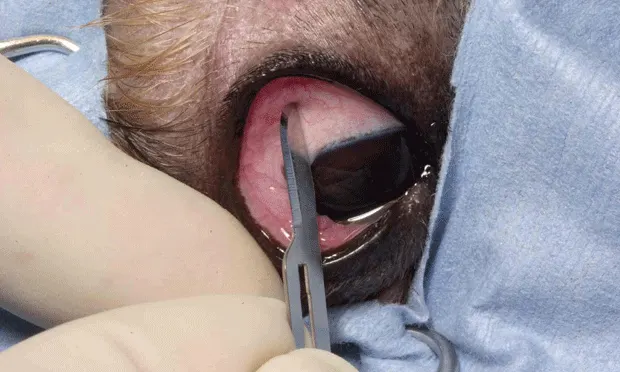
Position the eye and grasp the conjunctiva for biopsy using small-toothed forceps, such as a Bishop Harmon, or fixation forceps(A).Nontoothed forceps do not work as well as small-toothed-they cause the same level of discomfort but will not stabilize the tissue so it can be cut. Have a second assistant hold the patient's eyelid open. Grasp the conjunctiva gently with the forceps, lift up, and cut off a piece of tissue with tenotomy or iris scissors(B).This maneuver should be completed quickly and smoothly so the animal does not withdraw in reaction to pinching the tissue with the forceps. An awake dog or cat will not allow its tissue to be manipulated for an extended period, so plan the biopsy site and make sure the exposure is appropriate before beginning. Topical anesthetic provides partial anesthesia for the conjunctiva but the animal will feel pressure and cutting. Conjunctival tissue is very loose over the sclera and is easy to elevate so that a clean cut can be made without cutting the globe. This technique can be practiced on fresh cadaver eyes to get a feel for the elasticity of the conjunctiva.


Step 4
Bleeding is usually slight and can be controlled with topical 1/10,000 epinephrine drops, or topical 2.5% phenylephrine nasal or ocular drops. Suturing is not generally required because a small biopsy is sufficient.
Step 5
Roll or gently touch the sample to a glass slide to obtain impression smears for cytology (and Gram staining if a bacterial infection is suspected) and place the sample in a small tube of formalin for histopathology. Do not use too large a container or the sample will be lost in the volume of the preservative. Place the sample in a biopsy cassette to help prevent losing it and to help the pathologist identify the sample.
Procedure Pearl
Store proparacaine in the refrigerator after it is opened to maintain anesthetic potency.

Step-by-Step: Conjunctival Scraping for Cytology
Step 1
Restraint, lighting, and magnification are the same for conjunctival scraping as for biopsy. Make sure that the instrument used is sterile or from a cold sterilization tray and has been rinsed clean of the solution. A Kimura spatula, dental brush, or cotton swab will usually produce very nice cytology preparations. Before obtaining a scraping,
use a sterile culture swab to clean enough discharge from the eyelids and conjunctival sac to provide a clean area for sampling-saving some for culture (if needed). Using one hand, hold the eyelid open and expose the area in the conjunctival sac to be sampled. With the other hand, use the instrument (A-Kimura spatula) to scrape or brush (B & C-dental brush) the conjunctival surface.
Procedure Pearl
If the lesion is too fibrous or tough to obtain a sample with the spatula or brush, use a scalpel blade instead.



Step 2
If the lesion is too fibrous or tough to obtain a sample with the spatula or brush, use a scalpel blade instead. Start with the blunt end of the blade and switch to the sharp surface if needed to remove enough tissue for an adequate sample. In the photographs, the blunt end of the blade and the blunt side of the blade are being used.


Step 3
Gently roll or smear the instrument along a glass slide. Prepare at least 4 or 5 slides; our usual practice is to send 4 unstained slides to the clinical pathology laboratory for them to stain as they choose and to keep 1 slide to stain with Diff-Quik for our own evaluation.
Bleeding is usually not a problem after conjunctival scrapings, but if it occurs, treat as for a biopsy (see Step 4 in Step by Step Conjunctival Biopsy).
Procedure Pearl
Try to make the preparation very thin and don't press too hard or the cells will be crushed or damaged.


Selected Conjunctival Cytologic Samples
Sending cytology to a veterinary laboratory experienced in canine and feline conjunctival scrapings is a better practice than using a human laboratory unfamiliar with animal diseases. It is also essential that the veterinarian confer with the pathologist to ensure the pathologist is knowledgeable about ophthalmic conditions.

FIGURE A
Suppurative inflammation from confirmed herpesvirus conjunctivitis in a cat. Nondegenerate neutrophils, reactive epithelial cells, and a pigmented epithelial cell (Wright-Giemsa, ×250). Courtesy Rose Raskin and Atlas of Canine and Feline Cytology, 2001
Conclusion
Fractious or rambunctious patients will need sedation for these procedures. It is not important to keep the eye from rolling down when sampling the conjunctiva while the patient is under sedation, so any sedation protocol is appropriate as long as it is safe for the animal. We prefer medetomidine with butorphanol IV for young, healthy dogs and ketamine and diazepam IV for cats. Medetomidine also has the advantage of reversal with atipamezole. We are usually able to perform these procedures on nonsedated animals, unless the eye has a very deep ulcer and there is concern about rupturing the globe while restraining the patient.
Author's Note
Some photographs in this presentation show gloves and draping in use. To get good images, the patient was under general anesthesia. When the technique is performed on awake patients, draping or gloves are not required.