CBC is a routine diagnostic test used for health screening during annual physical examinations, etiologic investigation of clinical signs, confirmation of clinical suspicion, and monitoring patient response to treatment. Differential diagnoses are generated based on interpretation of laboratory results along with patient signalment, history, and clinical presentation; however, results can be unexpected, inscrutable, or inconsistent with clinical suspicion or other patient data. In these cases, spurious results secondary to sample handling, sample characteristics, and analyzer methodology limitations should be considered.
Following are, in the authors’ opinion, the 5 most common or important spurious results identified on CBC.
1. Pseudothrombocytopenia Caused by Platelet Clumping
Platelets have a primary role in hemostasis and should aggregate when vascular damage occurs.1,2 Venipuncture causes vascular damage that often results in platelet activation, aggregation, and clumping (Figure 1), especially when blood collection is difficult or a clean venipuncture is not achieved.1 Platelet clumping can occur when blood collection tubes are overfilled or inadequately mixed and is particularly prevalent in samples from cats.1-4
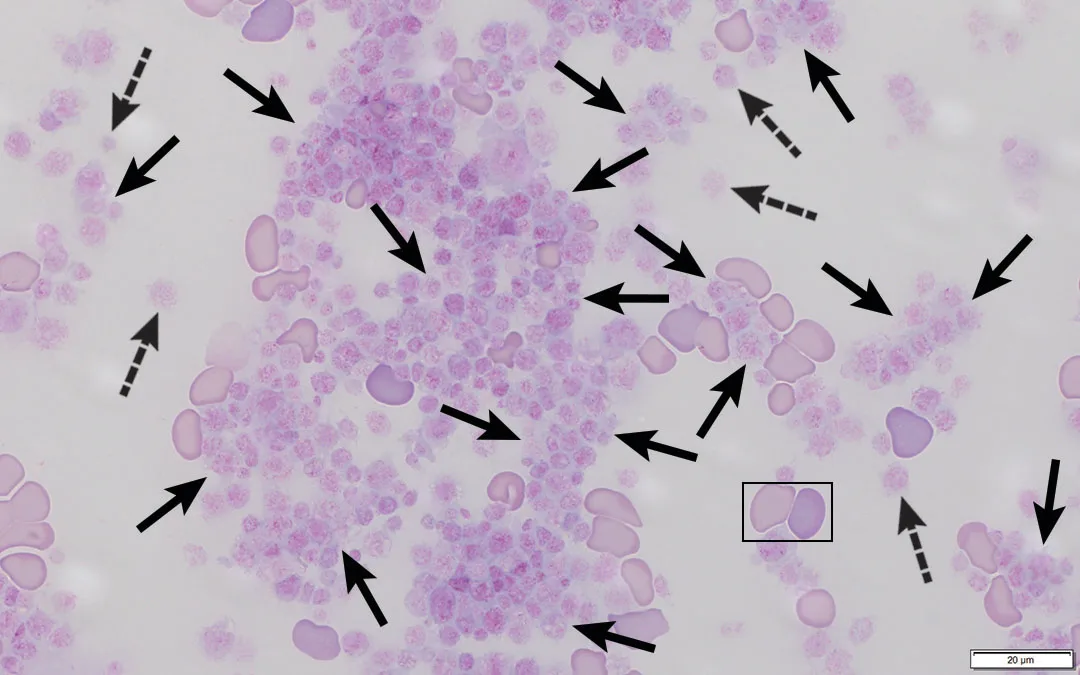
Large platelet clumps (solid arrows) in a blood sample from a dog. Microscopic clots or platelet clumps associated with overzealous platelet aggregation can result in pseudothrombocytopenia. Individual platelets (dashed arrows), a mature RBC (box, left), and a polychromatophil (box, right) can also be seen.
Pseudothrombocytopenia occurs when not all platelets in a sample are counted. Automated analyzers rely on either electrical impedance (ie, size) or light scattering properties to differentiate cell types. Neither of these methods reliably measure platelets in clumps; therefore, platelets are not counted individually, and falsely decreased platelet concentrations are reported by the analyzer.1,2,4,5
Remedy for Pseudothrombocytopenia Caused by Platelet Clumping
Microscopic examination of a blood film is essential to confirm thrombocytopenia and rule out pseudothrombocytopenia due to platelet clumps.1,2,4,5
Collection tubes should always be checked (eg, using 2 wooden sticks; Figure 2) for large clots before a sample is run through the hematology analyzer. Resampling may be indicated if gross evidence of blood clots is seen, with particular attention given to obtaining a clean venipuncture with smooth blood flow, as well as placing blood gently into the collection tube and mixing immediately.6

Blood clot (arrow) in an EDTA tube. Visual observation for large clots is recommended prior to analysis.
2. Falsely Increased Mean Corpuscular Hemoglobin Concentration Caused by Hemolysis or Lipemia
Mean corpuscular hemoglobin concentration (MCHC) is an indication of the average hemoglobin concentration in each erythrocyte in a sample.7
Only the sample hemoglobin concentration [Hgb], erythrocyte concentration [RBC], and mean corpuscular volume (MCV; typically) are directly measured by the analyzer.3 Calculated hematocrit (Hct) and MCHC are calculated from previously determined values.7 Calculated Hct is determined by the hematology analyzer using previously determined MCV and [RBC] (see Equation 1).7 MCHC is calculated by the analyzer using [Hgb] and calculated Hct (cHct; see Equation 2).
(1) cHct = (MCV × [RBC])/10
(2) MCHC = ([Hgb] × 100)/cHct
Understanding which values are measured directly, which values are calculated, and how the hematology analyzer determines reported values is important because interference or disturbance in directly measured values can also affect calculated values.8
Hemolysis (pathologic or in vitro) and lipemia (Figure 3) are common causes of spurious MCHC. With pathologic intravascular hemolysis resulting in hemoglobinemia, blood [Hgb] is used to calculate MCHC; however, [Hgb] includes both free hemoglobin in plasma as well as hemoglobin from erythrocytes, thus providing a falsely increased MCHC because [Hgb] is exaggerated compared with Hct.7 With in vitro hemolysis, blood [Hgb] is used to calculate MCHC, but the determined [Hgb] truly represents blood [Hgb]; however, Hct and RBC concentration are falsely decreased because of in vitro RBC lysis.7

Lipemic blood from an unfasted dog. This sample was refrigerated overnight, allowing RBCs to settle and thereby accentuate the lipemic nature of the plasma (arrow). Increased turbidity results in disturbances to hematologic assays that rely on light scattering (eg, plasma total protein via refractometry).
With hemolysis (whether pathologic or in vitro) and the resultant presence of free hemoglobin in plasma, there is a falsely increased MCHC because [Hgb] is higher than expected given the comparatively lower concentration of erythrocytes.3,7-9 Some correction methods are described in human medicine but no method has been validated in veterinary species.10
Lipemic samples cause inaccuracies in hemoglobin measurement that result in falsely increased MCHC because of lipid interference with plasma photometric properties.11 The analyzer confuses the lipid in lipemic samples with hemoglobin.
Remedy for Falsely Increased Mean Corpuscular Hemoglobin Concentration Caused by Hemolysis or Lipemia
External damage to erythrocytes can be minimized by ensuring there is minimal trauma during venipuncture and handling the blood sample gently after collection. The sample should be evaluated as soon as possible after collection. If analysis must be delayed, the EDTA blood sample can be stored in a refrigerator and evaluated within 24 hours. Whenever possible, a fasted blood sample should be used, or the effects of an unfasted sample should be considered. Plasma or serum color should be noted after centrifugation (from microhematocrit tube or separated serum for chemistry, if available).
3. Falsely Increased WBC Concentration Caused by Nucleated RBCs
Automated hematology analyzers use either electrical impedance (ie, size) or light scattering properties to determine the proportion of cell types.7,8 Nucleated erythrocytes (ie, nRBCs) are immature erythrocytes (ie, rubricytes, metarubricytes) that have not undergone nuclear expulsion during the maturation process.7,8,12 Nuclear presence results in the inclusion of nRBCs as part of the total WBC concentration ([WBC]) during analysis because nRBCs cannot be differentiated from leukocytes and the analyzer counts all cells with a nucleus as WBCs.3 Inclusion of nRBCs therefore results in a falsely increased WBC concentration.
In general, if the number of nRBCs per 100 leukocytes is >5, as determined during a manual differential count, the automated WBC concentration should be corrected.13 The acceptable threshold may vary slightly across reference laboratories.
Remedy for Falsely Increased WBC Concentration Caused by Nucleated RBCs
Microscopic examination of a blood film with a manual differential count should be performed. The correct total WBC with number of nRBCs per 100 cells should be counted (see Equation 3).
(3) 100/(nRBCs per 100 cells counted + 100) × automated [WBC] = corrected [WBC]
4. Incorrect Calculated Hematocrit Caused by Agglutination
Hct is typically determined via a manual, spun microhematocrit resulting in packed-cell volume (PCV) or calculation by an automated hematology analyzer to produce a calculated Hct (see Equation 1). With healthy patients and proper sample handling, PCV and calculated Hct values may differ slightly but should agree within 3 percentage points. A difference of >3 percentage points is referred to as an Hct mismatch and considered significant.7
Hct and PCV mismatch has many causes, including an inappropriately filled EDTA collection tube that results in too much EDTA for the amount of blood, an inappropriately mixed sample, or marked persistent hypo- or hypernatremia and agglutination.7 This article focuses on incorrect calculated Hct and Hct mismatch due to agglutination.
Agglutination is an immunoglobulin-complex–mediated, grapelike cluster or clump of erythrocytes and is most commonly associated with immune-mediated hemolytic anemia (IMHA).7 Immunoglobulin M molecule is a pentavalent (ie, 10 binding sites) antibody,5 resulting in the highest likelihood to form cohesive erythrocyte aggregates (Figures 4 and 5). Each large, cohesive erythrocyte cluster is counted as one larger RBC or may not be counted at all if the cluster is large, resulting in a falsely increased MCV and/or falsely decreased [RBC].9,11
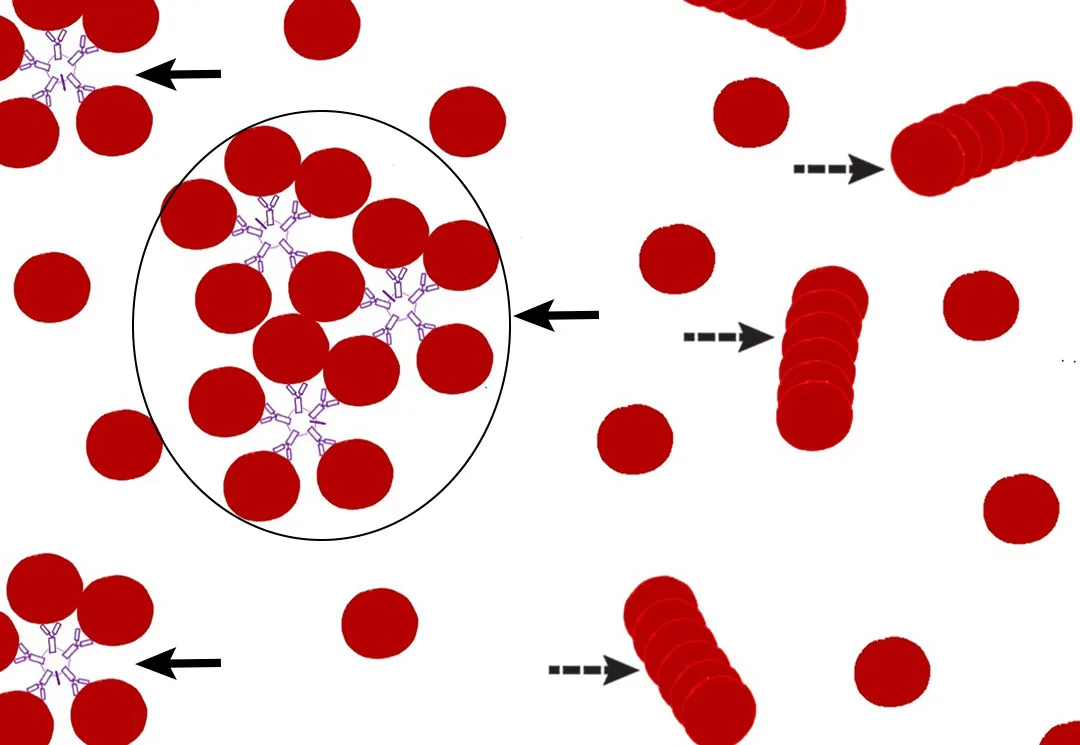
FIGURE 4
Schematic illustration of RBC agglutination (solid arrows) via the immunoglobulin M pentamer versus rouleaux (dashed arrows). The circled RBC cluster may be counted as one large RBC or not be counted.
Alterations in either MCV or [RBC] cause a spurious calculated Hct result because of how the analyzer calculates the Hct (see Equation 1), often producing an Hct mismatch. Falsely increased MCV causes calculated Hct to increase, and falsely decreased [RBC] causes calculated Hct to decrease. The resulting spurious calculated Hct could thus be either falsely increased or decreased to an unknown degree.7 In patients with agglutination, PCV (spun Hct) is thus a more accurate value than the calculated Hct provided by the analyzer.7
When considering the effects of agglutination, differentiating agglutination from rouleaux is also important.5 Rouleaux are typically described as stacking of RBCs because of electrostatic forces (Figure 4). Differentiation between agglutination and rouleaux on a blood film is not always straightforward.8 In such cases, a saline dispersion (ie, saline dilution) test may be helpful. The saline dispersion test involves diluting blood with saline, then immediately examining a wet-mount slide to determine the result. One drop of blood with 4 drops of saline is the recommended starting point, with a maximum dilution of 1 drop of blood and 9 drops of saline. Separation (dispersion) of the clumped erythrocytes is supportive of rouleaux. No dispersion (ie, RBC clumps remain) supports agglutination.7 Although rouleaux formation may be pathologically significant (typically a nonspecific indicator of inflammation in dogs and cats), unlike agglutination, rouleaux do not cause inaccuracies in calculated Hct.8
Remedy for Incorrect Calculated Hematocrit Caused by Agglutination
Microscopic examination of a blood film, adequate mixing of the sample before PCV determination or blood smear preparation, and a saline dispersion test to differentiate between rouleaux and true agglutination should be performed.14,15 With agglutination, spun PCV is a more reliable value than calculated Hct.7
5. Falsely Increased Plasma Total Protein Caused by Lipemia
Most reference laboratories include refractometric plasma total protein as part of the CBC. EDTA-whole blood is loaded into a microcapillary tube and centrifuged at high speed (ie, 13,000 × g) to separate the blood cells.7 Depending on the type of capillary tube used, plasma is either pushed from the tube directly, or, more commonly, the tube is broken just above the buffy coat and a drop of plasma is placed on the refractometer. The device uses light refraction properties to estimate the concentration of solids in plasma based on the assumption that the primary constituents are protein.16 Grossly lipemic plasma affects light refraction and falsely increases the measured protein value.5,16
Remedy for Falsely Increased Plasma Total Protein Caused by Lipemia
Whenever possible, patients should be fasted prior to blood sample collection. A sample from a nonfasted patient may have inaccurate analyzer results and should be interpreted accordingly.