Chronic & Persistent Coughing in a Dog
Douglas Palma, DVM, DACVIM (SAIM), The Animal Medical Center, New York, New York

Clinical History & Signalment
Louie, an 11-year-old, 53-lb (24-kg) neutered male Labrador retriever–poodle crossbreed, was presented for intense, productive coughing, gagging, and retching of 8 days’ duration. He had no known travel history or recent exposure to other dogs, and he was current on vaccinations and heartworm preventive. His owners reported no chronic respiratory signs, including voice change and/or coughing or gagging while eating or drinking.
Physical Examination
On physical examination, Louie was bright, alert, and responsive. Vital signs were within normal limits. His temperature was 101.3°F (38.5°C), heart rate was 80 bpm, respiratory rate was 30 bpm with normal effort, and capillary refill time was <2 seconds. Cardiac, pulmonary, laryngeal, and tracheal auscultation were all normal. No ocular or nasal discharge was present. Mild tracheal sensitivity was noted on direct palpation; abdominal palpation was normal. Several freely movable, homogeneous, subcutaneous masses were appreciated.
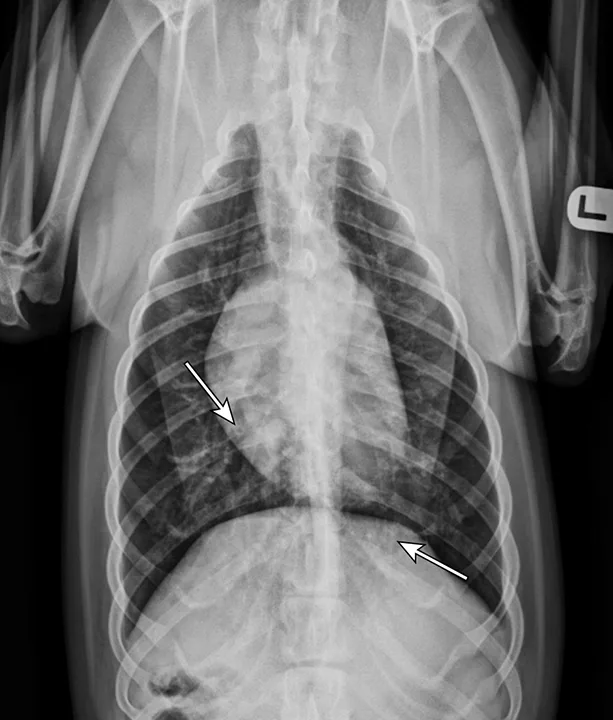
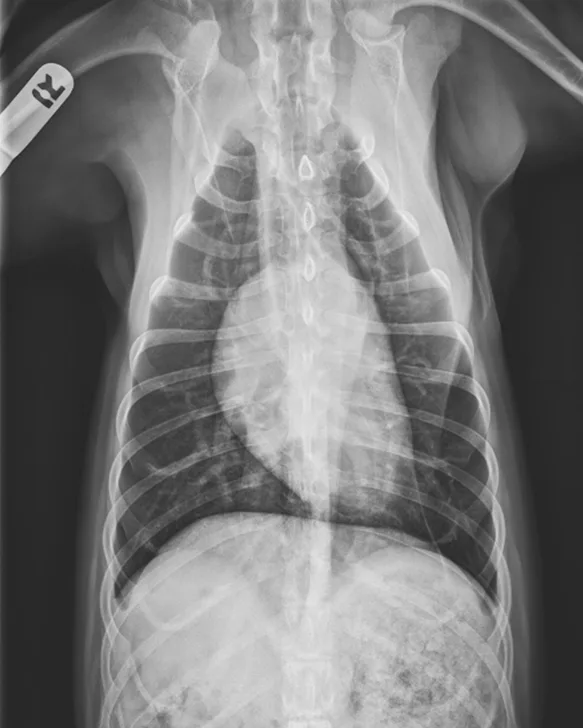
Thoracic radiographs showing a characteristic diffuse, patchy bronchointerstitial pattern (arrows)
Diagnosis
Differential diagnoses for acute onset cough should include canine infectious respiratory disease complex, pulmonary edema (cardiogenic or noncardiogenic), tracheal collapse, aspiration tracheitis/pneumonia, eosinophilic bronchopneumopathy (EBP), inhaled foreign bodies, and infectious disease (eg, fungal, protozoal, parasitic). Additional considerations include acute presentations of chronic processes, including bronchitis (eg, eosinophilic, chronic) or neoplasia.
Canine infectious respiratory disease complex was considered less likely based on lack of exposure to other dogs. Tracheal collapse and lung lobe torsion were of lower likelihood due to signalment, and fungal disease was considered less likely due to age and lack of exposure to enzootic areas. Heartworm disease was considered less likely based on chronic heartworm preventive administration, annual heartworm testing, and low geographic prevalence. Pulmonary edema was considered unlikely based on normal pulmonary auscultation, lack of heart murmur, and normal respiratory effort.
Thoracic radiographs revealed a moderate, diffuse, bronchointerstitial pattern (Figure 1). The cardiac silhouette, pulmonary vasculature, and extrathoracic structures were normal. Airway sampling via bronchoscopy was recommended based on radiographic findings. CBC and serum chemistry profile were performed prior to sedation. Serum chemistry results were within normal limits. CBC revealed leukocytosis (24.3 x 103/µL; normal range, 4.9-17.6 x 103/µL) characterized by marked eosinophilia (10.4 x 103/µL; normal range, 0.07-1.49 x 103/µL), monocytosis (1.4 x 103/µL; normal range, 0.13-1.15 x 103/µL), and band neutrophilia (729/µL; normal range, 0-170/µL). Heartworm antigen test was negative.
Bronchoscopic visualization revealed a moderate amount of thick, adherent, greenish-yellow mucus in the trachea and secondary and tertiary bronchi; mucosa was moderately irregular and erythematous (Figure 2). There was no evidence of airway collapse. Samples were collected via bronchoalveolar lavage for cytology, aerobic culture, and Mycoplasma spp culture.
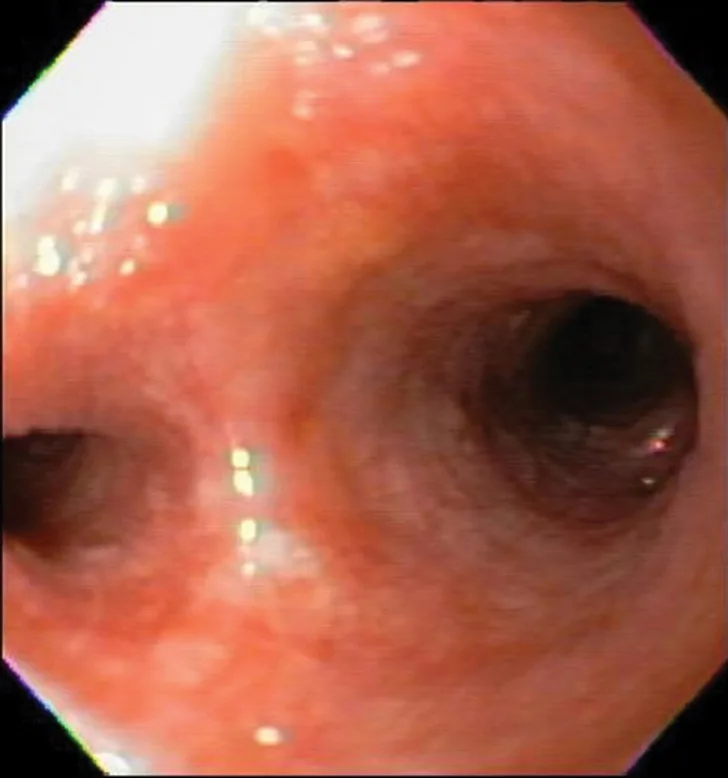
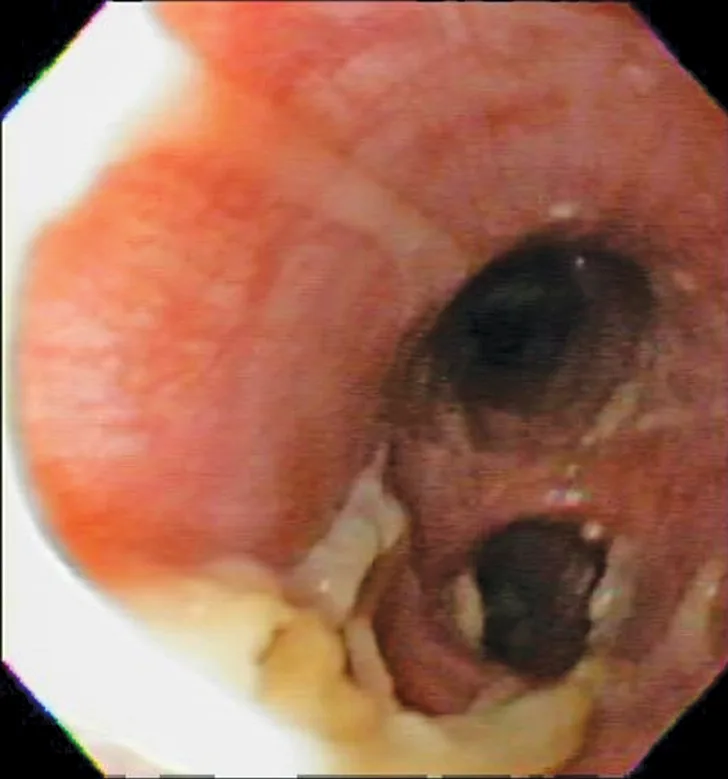
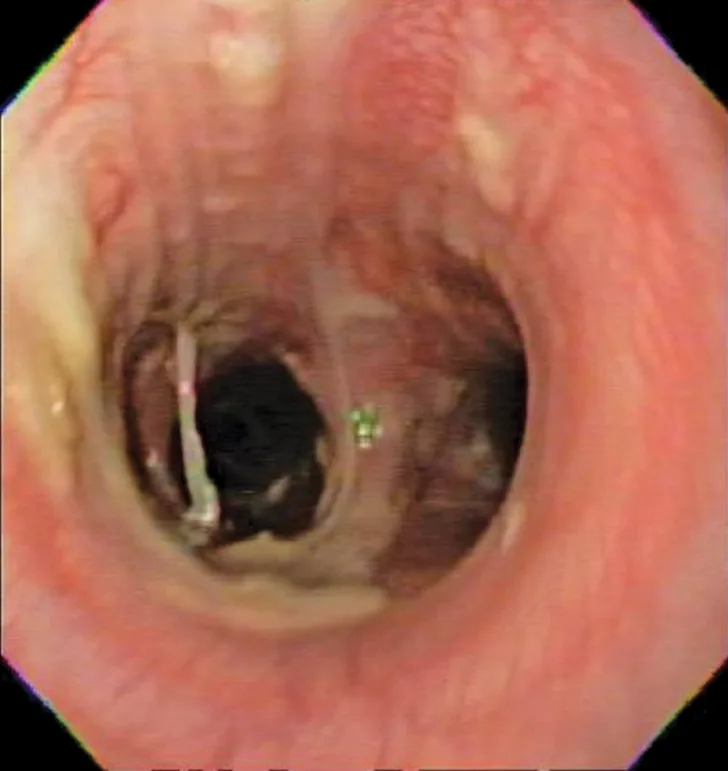
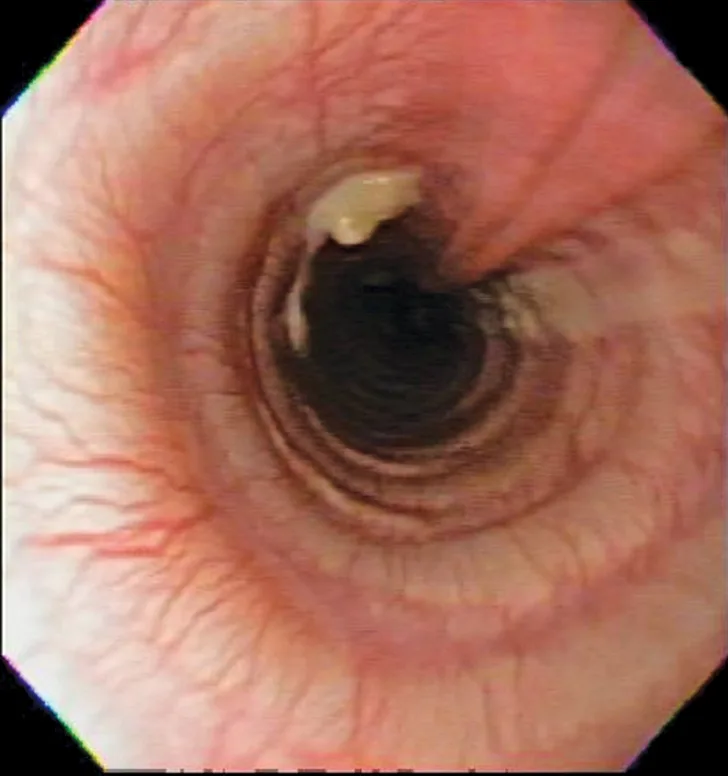
Bronchoscopy results demonstrating mucosal irregularity, hyperemia, and characteristic greenish-yellow airway exudate
Cytologic evaluation revealed a marked eosinophilic inflammatory response with no evidence of bacteria or sepsis; eosinophils made up 96% of total nucleated cells. The remaining cells were consistent with nondegenerate neutrophils (2%) and alveolar macrophages (2%) (Figure 3).
Mycoplasma spp culture was negative. Aerobic culture demonstrated small growth of Citrobacter freundii, Klebsiella pneumoniae, and Stenotrophomonas maltophilia, all of which were suspected to be contaminants in the absence of septic cytology.
A Baermann test and fecal centrifugation via zinc sulfate were performed because of the peripheral eosinophilia and eosinophilic cytology; results of both were negative. Repeat Baermann testing was not pursued due to the low clinical concern; however, repeat testing may increase sensitivity in populations with higher prevalence (ie, young dogs, immunosuppressed dogs, research dogs).
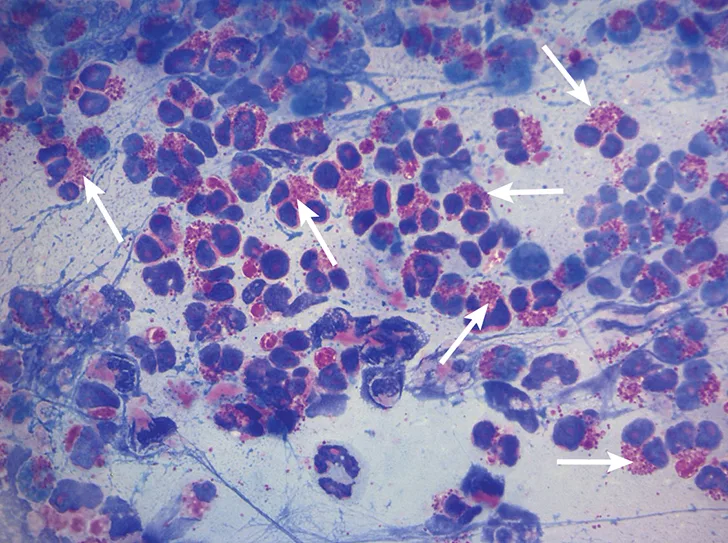
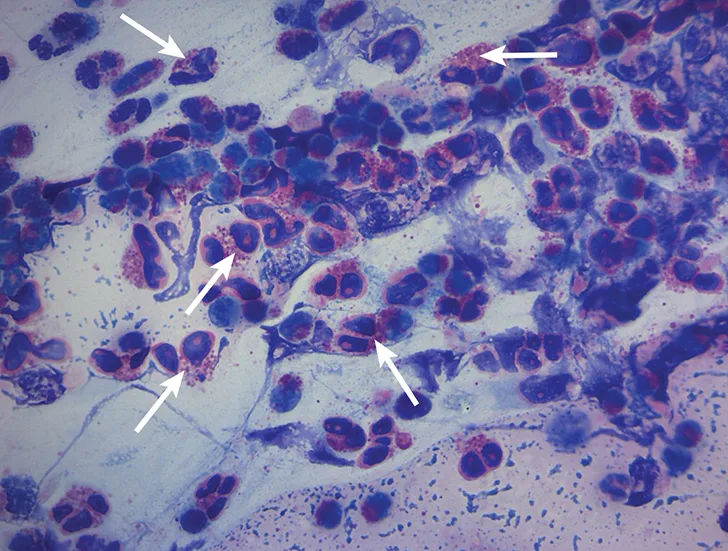
Characteristic cytologic eosinophilic inflammation. Pink granules typical of eosinophils can be seen (arrows).
DIAGNOSIS: Eosinophilic Bronchopneumopathy
Treatment & Long-Term Management
Treatment for EBP requires anti-inflammatory medications (see Treatment at a Glance). Louie was discharged on fenbendazole (1500 mg [50 mg/kg] once daily for 14 days) and prednisone (30 mg every 12 hours [2 mg/kg/day] for 5 days tapered to 20 mg every 12 hours [1.5 mg/kg/day] for 5 days; 15 mg every 12 hours [1 mg/kg/day] for 2 weeks; 10 mg every 12 hours for 2 weeks [0.67 mg/kg/day]; 10 mg every 24 hours for 2 weeks [0.33 mg/kg/day]; and finally 10 mg every 48 hours [0.33 mg/kg every other day]. Based on Louie’s clinical response, eventual discontinuation of steroids could have been considered.
Louie’s owner was asked to report any clinical signs (eg, coughing, gagging, retching) and corticosteroid adverse effects before each taper. Recheck examination and repeat radiography were recommended 1 month after discharge.
Treatment of inflammation should include corticosteroids, with or without immunomodulating medications, which should be gradually and slowly tapered. In endemic regions, concurrent treatment for parasitic disease (fenbendazole, 50 mg/kg for 10-14 days) should be considered to address migrating nematodes and/or primary pulmonary or tracheal parasites (eg, Paragonimus kellicotti, Filaroides spp, Crenosoma vulpis, Oslerus osleri), as false-negative results from traditional fecal testing are possible.
TREATMENT AT A GLANCE
Treatment should be provided for parasitic disease if necessary (ie, fenbendazole 50 mg/kg every 24 hours for 10 to 14 days.
Prednisone should be initiated at 1 to 2 mg/kg/day.
Prednisone should be tapered gradually, ideally every 1 to 2 weeks to the lowest effective dose.
Clinical signs should be monitored, and regular communication with the pet owner is recommended.
Radiography should be repeated to assess the patient’s response to treatment.
Relapse is common, and long-term therapy may be necessary.
Steroid adverse effects should be minimized; if these are excessive, alternative medications may include other immunosuppressants (eg, cyclosporine, azathioprine) and/or inhaled corticosteroids (eg, fluticasone, beclomethasone).
Prognosis & Outcome
At 1 week after discharge, Louie’s owner reported he had marked improvement in coughing (ie, ≈80% reduction) and increased energy; however, his owner also noted Louie had excessive thirst, urination, and appetite. At 2 weeks, his owner reported intermittent coughing and persistent signs of corticosteroid excess.
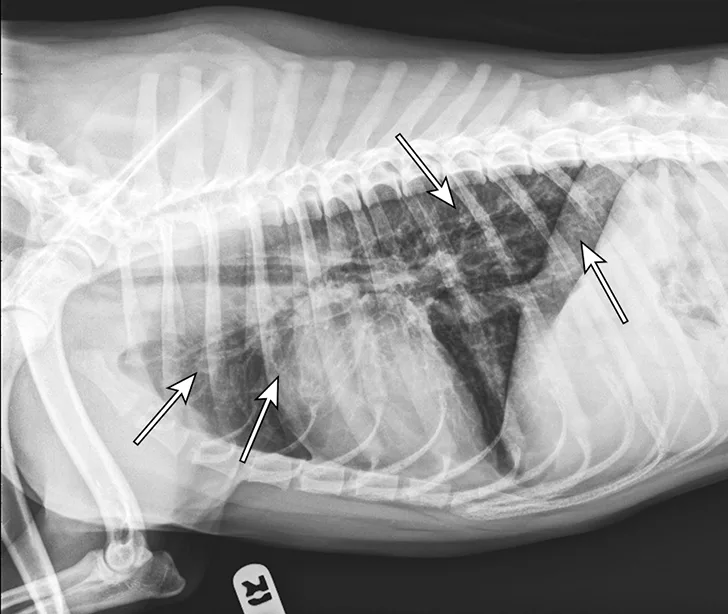
At 1 month after discharge, thoracic radiography was repeated and revealed marked improvement in diffusion of the bronchointerstitial pattern (Figure 4). Prednisone was tapered over the next 6 weeks to 10 mg every other day. Intermittent coughing returned, and the dosage was increased to 10 mg once daily (0.33 mg/kg/day), which maintained clinical control. Because this regimen did not result in significant adverse effects and maintained clinical control, adjunctive or alternative anti-inflammatory medications were not prescribed.
Louie was managed on a low dose of prednisone (10 mg once daily) for 5 years with no complications. The primary care clinician attempted to taper prednisone but was unsuccessful—coughing returned as the frequency of medication was reduced.
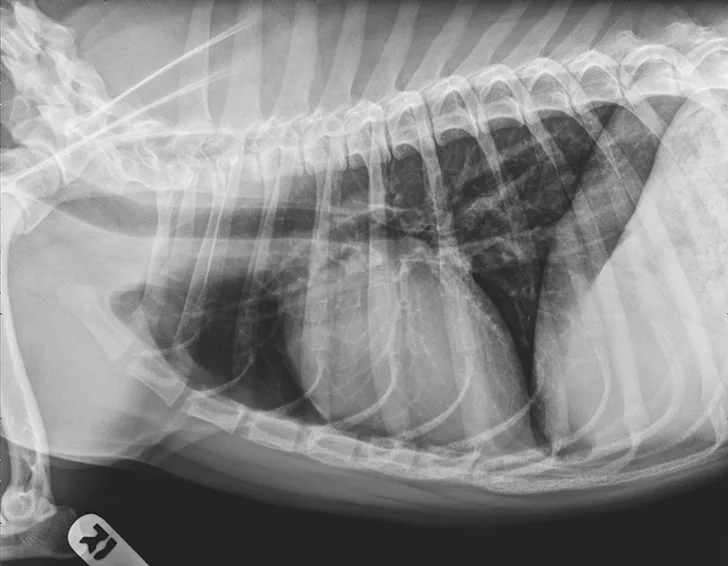
Radiograph 1 month after therapeutic initiation showing an improved bronchointerstitial pattern
Discussion
EBP (historically known as pulmonary infiltrates with eosinophils) is a disease characterized by eosinophilic infiltration of lung and bronchial mucosa and an important differential diagnosis for patients presented with chronic cough, acute onset of respiratory distress, and/or exercise intolerance.1,2 Cough is typically harsh and may be associated with gagging and retching.
This disease can be seen at any age; however, dogs 4 to 6 years of age are most commonly represented.1-3 Although Siberian huskies and Alaskan malamutes may be overrepresented,2 the disease can occur in any dog breed.
Physical examination findings may reveal abnormal lung sounds, but auscultation can be normal.1,2 In addition, ≤24% of patients may have concurrent nasal discharge.1,2,4
Diagnosis requires strong clinical suspicion, as EBP is an uncommon cause of the primary clinical signs (ie, coughing, gagging, exercise intolerance). CBC may reveal an inflammatory leukogram (eg, eosinophilia, neutrophilia) in ≤60% of cases.4 Eosinophilia may raise clinical suspicion, but its absence does not rule out disease, as it is only present in 50% to 60% of cases.1,2,5
Thoracic radiographs are generally characterized by a diffuse bronchointerstitial pattern with peribronchial cuffing and thickening of the bronchial walls. In some cases, bronchiectasis or alveolar infiltration may be observed.2,6-8 Occasionally, patchy pulmonary opacities create a nodular appearance.4 Radiography is critical for ruling out other common causes of cough and/or acute respiratory distress. Concurrent disease processes (eg, cardiomegaly, tracheal collapse) can complicate diagnosis.
EBP on thoracic computed tomography has been characterized by parenchymal abnormalities (93%) and bronchial wall thickening (87%) in most dogs. Many dogs also had mucus and/or debris that occluded the bronchial lumen (73%), lymphadenopathy (67%), or bronchiectasis (60%).9 Approximately 33% of dogs had pulmonary nodules, as has been identified on radiographs.4,9
Cytologic evaluation of the airways confirms eosinophilic inflammation, which is the hallmark of diagnosis. The percentage of eosinophils (mean, 61% of the total nucleated cell population4) exceeds that of healthy dogs (5%-24%).2,5,10 Samples can be obtained via tracheal wash or bronchoscopy. Bronchoscopy allows for visualization of more characteristic airway associated changes (eg, greenish-yellow secretions, irregular mucosa, hyperemia).2,5,11 Occasionally, intraluminal granulomas may be present,4 allowing for mucosal brush samples or biopsies that can further support a diagnosis. Tracheal washes provide appropriate cytologic samples in most cases. Bronchoscopy is generally reserved for patients with more focal radiographic disease, concerns for neoplasia, or suspicion for concurrent structural disorders (eg, bronchial collapse, tracheal collapse).
Cytologic evaluation is critical to help rule out parasitic disease that can also result in eosinophilic inflammatory response. Fecal testing (ie, Baermann test, fecal centrifugation) for parasitic disease is recommended; however, because negative results do not rule out parasitic disease, repeat testing and/or empirical therapy is advisable, particularly in at-risk patients.12 Heartworm testing is also indicated because heartworm disease may be associated with pneumonitis and eosinophilic inflammation (Table).13
COMMON FINDINGS IN EOSINOPHILIC BRONCHOPNEUMOPATHY
Eosinophilic bronchitis (EB), eosinophilic granuloma (EG), and EBP have been retrospectively evaluated and may provide information regarding therapeutic response, indications for chronic therapy, and overall prognosis.4 EB is associated with less severe airway remodeling, reduced eosinophilic inflammation (ie, percent of nucleated cells in airway samples), reduced total inflammation (bronchoalveolar lavage total nucleated cell count/µL), and lower incidence of peripheral eosinophilia. Patients with EG demonstrate intraluminal granulomas/masses not present in EBP. Overlap still exists among these disorders, and the impact on therapeutic outcome is not yet known; however, EB may be more responsive and EG less responsive to therapy.4 Eosinophilic pulmonary granulomatosis may also be associated with eosinophilic inflammation, but it is least common and may represent a spectrum of disease characterized by masses that involve the pulmonary parenchyma (not exclusively luminal). EPG may also have systemic organ involvement and be the least responsive to therapy.14,15
Corticosteroids are the mainstay of therapy. Prednisone is typically initiated at 1 to 2 mg/kg/day and gradually tapered to the lowest effective dose.1,2,4 It is important to note that clinical relapse is common and can reach ≈30% after corticosteroids are tapered.5 In some cases, medications can be discontinued after months with no evidence of relapse. Some clinicians may taper the drug faster, but the author believes that tapering faster than 1 to 2 weeks may increase the rate of relapse.
Patients with severe signs (eg, hypoxemia, oxygen dependency, alveolar disease) may also benefit from higher doses initially.
Alternative options for management include inhaled corticosteroids or additional immunosuppressive medications. Fluticasone has been evaluated and may be successful in some cases but is not always adequate for maintaining clinical remission.16 The author has successfully used cyclosporine in some cases in which corticosteroid adverse effects were intolerable. However, cyclosporine and other immunosuppressive drugs (eg, azathioprine, mycophenolate) have not been evaluated in randomized, controlled studies despite sporadic clinical use. Cyclosporine has been used for the treatment of other eosinophilic disorders in dogs.17,18
TAKE-HOME MESSAGES
EBP should be considered in patients with chronic cough and in patients with acute respiratory distress.
Radiographs may reveal nodules, mimicking neoplasia.
Absence of eosinophilia does not rule out the disease.
Diagnosis requires cytologic evaluation.
Parasitic disease can mimic EBP and cannot be definitively ruled out with negative testing.
Corticosteroids (ie, prednisone) are the hallmark of therapy.
Patient relapse is common and may necessitate long-term management.
Inhaled steroids may not always control the disease.