Canine Pancreatitis: A Complete Guide
PROFILE
Definition
Pancreatitis (ie, inflammation of the pancreas) can be acute, chronic, or acute on chronic.
Systems
Effects range from mild GI signs (eg, decreased appetite, occasional vomiting) to systemic inflammatory response syndrome (SIRS) and multiple-organ dysfunction syndrome.
Incidence & Prevalence
Increasing diagnostic sensitivity and specificity may result in an increased incidence of pancreatitis diagnosis.
Related Article: Is This Pancreatitis?
SIGNALMENT
Breed Predilection
Any breed can be affected.
Several breeds (eg, schnauzer, Yorkshire terrier, spaniels, boxer, Shetland sheepdog, collies) are overrepresented.1
Whether genetic mutations of serine protease inhibitors in schnauzers contributes to the development of pancreatitis has not been determined.
Age & Range
Typically affects middle-aged to older patients that may be overweight or have history of dietary indiscretion
Causes
Underlying causes are poorly understood.
Several veterinary medications have been implicated to cause pancreatitis.
Dietary indiscretion (± high-fat content) and some toxins (eg, zinc, castor beans) are generally accepted causes.
Other causes include pancreatic ischemia (result of hypotension from fluid loss or anesthesia); surgical manipulation (poorly described); biliary, pancreatic duct, and intestinal disease; and pancreatic trauma.
The inciting cause may be idiopathic.
Risk Factors
Patients that are obese or have other endocrine disease or systemic illness may be at risk.
Hyperlipidemia, diabetes mellitus, hypothyroidism, and hyperadrenocorticism have been associated with pancreatitis.
Whether these are comorbidities or causing factors is unknown.
Pathophysiology
Results from activation of potent pancreatic enzymes and local and systemic consequences of the ensuing inflammation.
Several homeostatic mechanisms prevent intrapancreatic activation of these enzymes.
In healthy states, these enzymes are stored as zymogens in an inactive form and segregated in the endoplasmic reticulum.
When enzymes are abnormally activated in the pancreas, the ensuing proteolysis activates the inflammatory cascade and production of free radicals and phospholipase, which can disrupt cellular membranes, cause cytokine (eg, TNF-α, interleukin-1) production, and result in subsequent neutrophil recruitment and exacerbation of the inflammatory cascade.
In severe cases, this may result in systemic consequences (eg, SIRS) with devastating effects: focal or diffuse peritonitis, respiratorydifficulty (eg, acute respiratory distress syndrome [ARDS]), renal injury, hepatobiliary dysfunction, and coagulopathic disease (eg, disseminated intravascular coagulation [DIC]).
Local inflammation can lead to increased capillary permeability, edema, necrosis, and hemorrhage.
Related Article: New Tests for Pancreatitis
History, Physical Examination, & Clinical Signs
Common presenting complaints include decreased appetite or anorexia, lethargy, vomiting, diarrhea, and abdominal pain.
Examination findings are often nonspecific but may include evidence of nausea (eg, lip licking, ptyalism, regurgitation/vomiting with abdominal palpation, eructation), dehydration, altered gut sounds (increased/decreased borborygmi), abdominal pain, fever, icterus, and hypovolemic shock.
Similar signs may result from other causes of acute abdomen: gastroenteritis, hemorrhagic gastroenteritis, toxin ingestion, hepatobiliary disease, primary infiltrative or obstructive GI disease, renal disease, lower urinary tract disease, liver failure, organ torsion.
DIAGNOSIS
Histopathologic examination of the pancreas is the gold standard.
Most patients do not require surgery, and diagnosis is often based on historical and physical examination findings with clinical pathology and abdominal imaging.
However, many of these findings have relatively poor sensitivity and/or specificity for pancreatitis.
Laboratory Findings
CBC data for diagnosis include:
Elevated PCV from dehydration
Inflammatory leukogram (± left shift)
Thrombocytopenia
Serum biochemistry profile abnormalities may include mild-to-moderate elevation of cholestatic or specific hepatocellular liver enzymes and bilirubin.
Electrolyte and blood gas abnormalities are often secondary to fluid loss from vomiting (eg, hypochloremic/ hypokalemic metabolic alkalosis).
Azotemia can be present and is most commonly associated with prerenal dehydration, which may also be reflected in elevated total protein.
Hypoalbuminemia may result from GI losses, potential third space fluid accumulation, and/or development of peritonitis; albumin is a negative acute-phase protein.
Lipase and amylase have poor sensitivity and specificity for pancreatitis (amylase, 14%–73%; lipase, 18%–69%).
Commercial laboratory and point-of-care canine pancreas-specific lipase (cPLI) tests have demonstrated sensitivity of ≥82% and specificity of 96% for diagnosing pancreatitis, although false-negative and false-positive results may occur.1
The sensitivity of other diagnostic testing is much lower: trypsin-like immunoreactivity (cTLI) has a sensitivity of 36%–47% and abdominal ultrasonography, 68%.1
Further studies comparing cPLI with histopathologic and imaging findings are warranted.
Additional routine diagnostics (eg, urinalysis) may be necessary.
Secondary systemic complications may indicate coagulation times, blood gas analysis, urine culture, and cytologic and clinicopathologic evaluation of abdominal fluid (if present), along with thoracic radiography.
Related Article: A Tail of Triaditis
Figure 1. Ultrasound of the right upper quadrant of a dog’s abdomen showing changes often noted with pancreatitis (eg, thickened, hypoechoic pancreas [yellow arrow], surrounding hyperechoic mesentery [white arrow]).

Imaging
Abdominal radiographic findings are usually nonspecific but may demonstrate detail loss or ground glass appearance in the right upper quadrant and a wide angle between the duodenum and stomach antrum.
Ultrasonography (See Figure 1) remains one of the most common methods to diagnose pancreatitis.
A skilled ultrasonographer can often identify characteristic ultrasonographic changes consistent with pancreatitis (eg, enlarged hypoechoic or mixed echogenic pancreas with surrounding hyperechoic mesenteric tissue, variable distention/functional obstruction of the biliary system, small amounts of free fluid in the abdomen consistent with focal peritonitis, thickened or corrugated appearance to the duodenum, intestinal ileus).
A normal ultrasound does not rule out pancreatitis.
Advanced imaging (eg, CT) is likely more sensitive but is often not pursued because of cost.
Other Diagnostics (if Applicable)
Laparoscopically obtained biopsy may be an alternative to celiotomy for gathering histopathologic evidence of pancreatitis when other imaging techniques are unavailable or unclear.
Figure 2. Radiograph confirming appropriate postoperative placement of a nasogastric tube in a dog.

TREATMENT
Inpatient or Outpatient
Inpatient or outpatient treatment is largely based on severity of clinical signs.
Patients that fail outpatient therapy should be hospitalized.
The mainstay of therapy is to treat or eliminate the underlying cause and provide symptomatic and supportive care.
The author recommends Kirby’s Rule of 20 to monitor patient requirements.2,3
COP = colloid osmotic pressure, cPLI = canine pancreas-specific lipase, cTLI = canine trypsin-like immunoreactivity, SIRS = systemic inflammatory response syndrome

Figure 3. Nasogastric tube sutured to the nasal philtrum (3A) and to the skin ventral to the zygomatic arch (3B).
Medical
Crystalloid fluid supplementation should be used to correct perfusion deficits and dehydration with ongoing supplementation to account for maintenance and continued losses.
Patients with fevers have mildly increased fluid requirements (~7% more than normal for each degree).4
Colloid management with hydroxy-ethyl starches is often used to supplement patients with SIRS presentations (that lead to capillary leak and protein losses) to help maintain colloid osmotic pressure (COP).
Potassium supplementation is often necessary.
Administration of fresh frozen plasma has shown no benefit.
Analgesic therapy may improve appetite, ventilatory capacity, and mobility.
Opioid analgesics (eg, fentanyl, methadone, hydromorphone (See Table) may help resolve abdominal pain.
Infusions of ketamine and lidocaine or local therapies (eg, epidural injections) may be used to treat pain.
NSAIDs and steroids may exacerbate GI ulceration, renal injury, and pancreatitis.
Antiemetics for vomiting or nausea are commonplace; newer available drugs (eg, maropitant, dolasetron, ondansetron (See Table) can decrease vomiting.
Recent studies have shown that maropitant is more effective than metoclopramide and chlorpromazine.5,6
Proton pump inhibitors (pantoprazole) or histamine-2 receptor antagonists (ie, famotidine) and sucralfate can help treat associated gastric ulcers.
Because most pancreatitis cases are not associated with bacterial infection, antibiotic therapy is rarely warranted.
However, select cases may benefit from antibiotics.1
Plasma transfusions (to deliver colloid support via antitrypsin/antiproteases) have shown little benefit and can be costly.
Nutritional
Nutritional supplementation is paramount to recovery.
Little evidence supports outdated therapies involving no PO food or water in patients with pancreatitis (to supposedly rest GI systems).
Villous atrophy occurs within hours of discontinuing oral alimentation and may prolong recovery if not addressed early.
Early enteral nutrition is often well tolerated with few complications.
This may improve gut barrier function and decrease bacterial translocation.
Easy-to-place tubes (eg, esophagostomy tubes) are well tolerated, allow enteral nutrition, and are associated with few complications.
Nasogastric tubes allow aspiration of gastric contents, which may decrease nausea and vomiting and help prevent aspiration pneumonia (See Figures 2 and Figure 3).
Trickle feeding theoretically helps bypass cephalic, gastric, and intestinal phases of pancreatic secretion.
Nasoesophageal tube feeding is an alternative but does not allow gastric suctioning.
Jejunal or gastrostomy tubes require endoscopy or surgery to place and may be more expensive but should be considered for surgical intervention.
Total parenteral nutrition may provide adequate calories and be useful in select cases but requires strict aseptic delivery and does not promote GI (villous) recovery.
Surgical
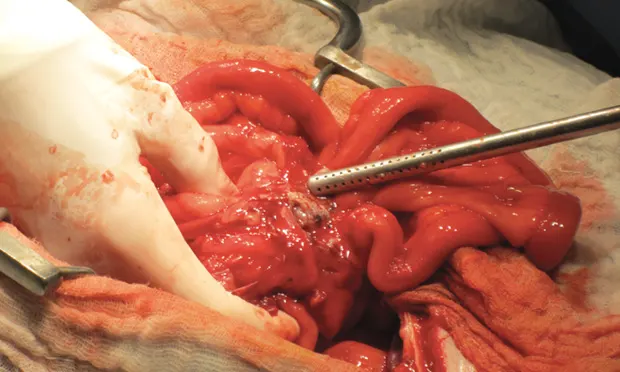
Surgical treatment is rarely indicated but may be necessary if the diagnosis is unclear or in patients that develop extrahepatic biliary obstruction, pancreatic abscessation, or peritoneal sepsis or that deteriorate despite aggressive therapy (See Figure 4).
Surgical procedures may include pancreatic or peritoneal lavage, debridement of necrotic tissue, drainage, partial pancreatectomy, and placement of a feeding tube.
Figure 4. Surgical exploration of a dog with a pancreatic abscess (arrow); note the diffuse moderate erythema/peritonitis.

IN GENERAL
Relative Cost
Many patients can be managed with outpatient therapy (eg, SC fluids, antiemetics, pain medication): $
Mild-to-moderate cases may require hospitalization with IV fluids and pain medication, management with a temporary feeding tube, or IV nutrition: $$$$
Severe cases (eg, biliary obstruction, pancreatic neoplasia, abscessation) may require surgical intervention: $$$$$
Severe cases can develop secondary systemic consequences, as pancreatitis is an inciting cause of SIRS and may require multiple days in hospital with aggressive supportive care: $$$$$
Prognosis
Prognosis for both acute and chronic pancreatitis is good.
Severe cases can lead to euthanasia because of cost or because of multiple-organ failure, sepsis, SIRS, ARDS, and DIC (all rare).
Future Considerations
Clients should be informed that pancreatitis is on a continuum; patients may have chronic pancreatitis or intermittent bouts of acute pancreatitis that require long-term management.
Chronic or recurrent acute on chronic pancreatitis may result in systemic consequences (eg, exocrine pancreatic insufficiency, diabetes mellitus from pancreatic fibrosis).
ARDS = acute respiratory distress syndrome, DIC = disseminated intravascular coagulation, SIRS = systemic inflammatory response syndrome
ANDREW LINKLATER, DVM, DACVECC, directs management of critical patients, performs advanced procedures, and provides lectures and instruction to students, interns, and residents at Lakeshore Veterinary Specialists in Glendale, Wisconsin. His interests include trauma, surgical emergencies, mechanical ventilation, and transfusion medicine. Dr. Linklater completed an internship in Los Angeles and a residency in emergency and critical care at Animal Emergency Center in Milwaukee, Wisconsin. He graduated from University of Saskatchewan Western College of Veterinary Medicine. Dr. Linklater has publications in journals and textbooks and has lectured nationally and internationally, including at the NAVC Conference.