Canine Heartworm
Andrew R. Moorhead, DVM, MS, PhD, DACVM (Parasitology), North Carolina State University
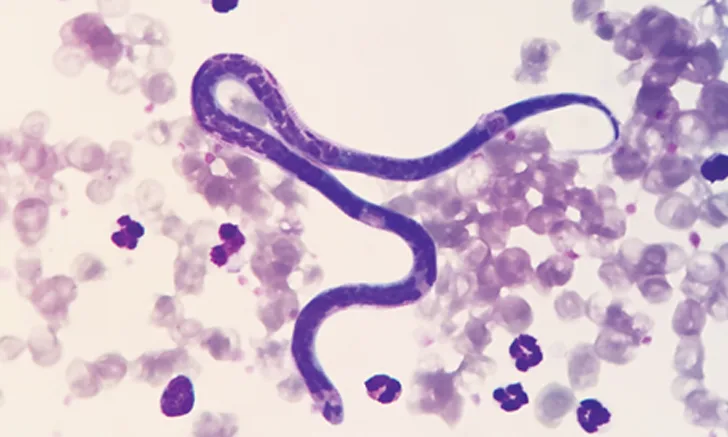
Dirofilaria immitis (ie, canine heartworm), a potentially deadly disease, is arguably the most important parasite that affects dogs in North America, with ≈100,000 new cases reported annually.1 It is thus important that all clinicians (including those in historically nonendemic regions) be knowledgeable regarding the heartworm life cycle, as this will allow for better understanding of treatment and prevention strategies.
Life Cycle
Adult female heartworms can grow to a length of 10 to 12 inches; male heartworms typically reach 4 to 6 inches. Adult worms can live up to 5 to 7 years in dogs, where they mate and produce microfilariae (>300 µm in length) that circulate in the blood. Microfilariae are then ingested by an intermediate mosquito host—this is essential for heartworm development—in which they migrate for an average period of ≈14 days and develop to first-stage larvae (L1). The L1 then molt twice and become infectious third-stage larvae (L3) that are ≈1 mm in length and exist in the head of the mosquito. The ambient temperature must be >57.2°F (14°C) for larval development in the mosquito.2 When the mosquito lands and takes a blood meal, the L3 emerge from the proboscis (ie, mosquito mouthparts) and surround the stylet (ie, piercing part of the proboscis) in a pool of mosquito hemolymph (Figure). When the stylet is removed, the larvae enter the host through the hole created by the stylet. This process is in contrast to the commonly held belief that larvae are injected by the mosquito into the definitive host.3
Once inside the definitive host, the larvae follow a complicated migration pathway. The L3 remain at the site of entrance for ≈3 to 4 days; during which time they molt to fourth-stage larvae (L4). Molting is usually completed within 4 days but may not occur until day 12.3 L4 typically molt to the last stage (ie, juvenile adults) between day 50 to 58, then migrate through the subcutaneous tissue and musculature.4 Worms begin arriving in the pulmonary artery (ie, the final location) by days 67 to 704,5; most worms reach this location by day 120. By day 180, worms are sexually mature and begin to produce microfilariae; thus, completing the life cycle.4 This timeline can vary.
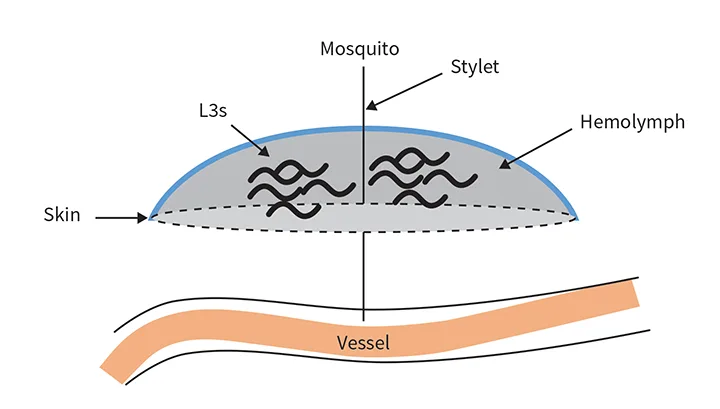
L3 in a hemolymph pool, with the mosquito stylet still inserted in the definitive host
Pathophysiology
Heartworm disease can result in death, especially in cases with a large number of worms (ie, caval syndrome). Although this acute presentation can be remarkable, pathology is most often observed secondary to the prolonged presence of worms in the pulmonary arteries, which can occur as early as day 60 to 70 of infection.4,5 Severity of disease and extent of pathology are influenced by multiple factors (eg, number of adult worms, duration of infection, host immune response, presence of dead worms). The presence of dead worms is especially important, as they are carried by the blood flow further into the pulmonary vessels, resulting in pulmonary thromboembolism (PTE).
Heartworms are constantly pushed back and forth by the pulsing blood flow; this can cause trauma to the vessels that results in thickening of the tunica intima and inflammation of the vessel wall characterized by a pathognomonic roughened, stippled appearance.6,7 This prolonged damage can eventually lead to vessel inelasticity, resulting in increasing pressure on the main pulmonary artery, right heart, and vena cava. In turn, this pressure can lead to chronic, passive congestion and pulmonary hypertension that results in right-sided heart enlargement and (eventually) failure.8 Right-sided heart failure can cause liver congestion and ascites. In addition, renal lesions (eg, glomerulonephritis) can develop secondary to immune complex deposition.9 Damage increases with the persistence of worms in the vessels.
Although worms can cause damage, data suggest that endosymbiotic Wolbachia spp can cause some of the inflammation associated with heartworm disease, specifically after worm death. These bacteria are endosymbionts of many filarial worms, are transmitted vertically, and live within the worm; they do not result in overt pathology.10 When the worms die (either of a natural cause or due to drug treatment), the surface proteins of Wolbachia spp are exposed to the host, and the host’s immune system responds.11 It is therefore important to eliminate Wolbachia spp from the worms before treatment is initiated.
Clinical Signs
Dogs infected with D immitis may be presented with no to mild, moderate, or severe clinical signs or with caval syndrome. Although the severity of clinical signs depends on many factors, the presence or absence of clinical signs is important for staging the disease (see Clinical Signs of Heartworm Disease).
CLINICAL SIGNS OF HEARTWORM DISEASE
No to mild signs: Patients are subclinical or have a mild cough but otherwise appear healthy.
Moderate signs: Patients have a moderate cough, can have difficulty breathing, and can be slightly exercise intolerant (eg, inability to run, tire more easily than normal on walks).
Severe signs: Patients are dyspneic and can be severely exercise intolerant (eg, difficulty walking or walking with labored breathing). Syncope and hemoptysis may be present. Signs of right-sided congestive heart failure (eg, ascites) are present.
Caval syndrome: Patients typically have associated acute presentation of signs (eg severe dyspnea, collapse) normally related to a large numbers of worms obstructing blood flow through the tricuspid valve. Hemoglobinemia and pigmenturia are characteristic. Onset is rapid and results in death after 12 to 72 hours if not treated.8
Diagnostics
A 3-dose melarsomine treatment protocol is safe,8 but complications are possible. Disease staging should thus be performed prior to treatment. Diagnostics should include physical examination, immunodiagnosis, microfilariae testing, radiography, and cardiac ultrasonography, as well as CBC, serum chemistry profile, and urinalysis. However, if diagnostic staging cannot be performed, the 3-dose protocol is recommended over other treatment protocols.
Physical Examination
Physical examination should occur first and clinical signs indicative of disease severity (see Clinical Signs of Heartworm Disease) should be assessed.
Immunodiagnosis
Antigen testing is considered the gold standard for diagnosing heartworm disease. Antigen tests are highly sensitive and specific for infections of adult female worms >8 months of age because older worms tend to produce more antigens. However, single-sex infections of only males are undetectable using this method. Antigen tests do not routinely detect prepatent infections (worms <5 months of age) and are not quantitative. The color of any test is not correlated with worm burden. Antigen tests can also be used to determine the effectiveness of adulticidal treatment. If all worms are killed, adult antigens should be cleared from the blood by 9 months after treatment.8
In a subset of dogs with adult female heartworms, no detectable antigen is present. This may be due to immune complex formation between a heartworm-derived antigen and antibodies against the antigen. Therefore, it is crucial to test all dogs for microfilariae at the same time as antigen testing in case the antigen test is negative due to sequestration of the antigen by immune complex formation.12 Immune complexes can be dissociated with heat treatment of the sample. Current recommendations for heat treatment of serum/plasma are when no antigen is detected but the patient is microfilaremic and/or has clinical signs.
Microfilariae Testing
Several methods can be used to evaluate for the presence of microfilariae, regardless of antigen status. Examination of a drop of blood or direct smear detects fewer infections than a concentration technique (eg, Knott or filter test). For the aforementioned reasons regarding the formation of immune complexes, microfilarial testing should be performed simultaneously with antigen testing. Because as many as 20% of dogs may be amicrofilaremic, this is not recommended as a stand-alone diagnostic test.8
In addition, D immitis should be differentiated from Acanthocheilonema (Dipetalonema) reconditum, which is a nonpathogenic worm that is transmitted by fleas, lives in the subcutaneous tissue, and does not require treatment. The easiest way to differentiate these microfilariae in a clinical setting is to observe their movement under a microscope. A reconditum moves progressively, whereas D immitis are mobile but remain in a single location.
Radiography
Radiography enables assessment of damage that has already occurred.
Cardiac Ultrasonography
ECG is not a first-line diagnostic method for heartworm disease. However, visualization of heartworms via ultrasound can confirm infection; lack of visualization does not rule out infection.
CBC, Serum Chemistry Profile, & Urinalysis
Liver and kidney function should be assessed before administration of melarsomine. Existing disease should be considered prior to treatment.8
Treatment
Previously, intravenous thiacetarsamide sodium was used to treat D immitis, but this drug had serious adverse effects.13 Later, melarsomine dihydrochloride was introduced and considered to be a significant improvement for treatment, with early treatment protocols involving a straightforward 2-dose injection protocol and the 3-dose protocol being reserved for more complicated cases.14 Due to advancements in knowledge about D immitis, treatment protocol now involves additional components, including melarsomine dihydrochloride, macrocyclic lactones, corticosteroids, and doxycycline.
Protocol
The timing of each component of heartworm disease treatment is detailed by the AHS.8 Macrocyclic lactone and 4-week doxycycline treatment should be initiated at the time of diagnosis. After 1 month and then monthly, a macrocyclic lactone should be administered unless a sustained-release moxidectin product was chosen as the macrocyclic lactone in the beginning of the treatment protocol. Two months after diagnosis, the first dose of melarsomine should be administered, followed 1 month later by a second and third dose given 24 hours apart. It is thought that the month in between the end of doxycycline and the start of melarsomine therapy is necessary for worm-mediated degradation of Wolbachia spp killed by doxycycline,15 ensuring no immunogenic Wolbachia spp surface proteins are released into the bloodstream. Because Wolbachia spp is an endosymbiont, killing the bacteria should also weaken the worm, as Wolbachia spp is required for worm survival.
Macrocyclic Lactones
Macrocyclic lactone preventive treatment should be started at the time of diagnosis (ie, 2 months prior to the first melarsomine treatment). This can help prevent further infections and help eliminate developing larvae.
Doxycycline
The antibiotic doxycycline (recommended dosage, 10 mg/kg every 12 hours for 28 days) is critical in the treatment of heartworm disease because of the drug’s activity against Wolbachia spp.8
Melarsomine Dihydrochloride
The labeled dosage for melarsomine is 2.5 mg/kg IM twice in the epaxial muscles 24 hours apart.8 However, the AHS recommends an alternate or 3-dose regimen, in which 1 injection (2.5 mg/kg) is administered, then 2 doses are given 30 days later at a 24-hour interval. This regimen has increased safety and efficacy but includes an additional month of exercise restriction, increased total arsenical dose, and additional cost of a third injection.8
Corticosteroids
Prednisone (0.5 mg/kg every 12 hours the first week, 0.5 mg/kg every 24 hours the second week, 0.5 mg/kg every 48 hours the third and fourth weeks) can be administered.8 It is recommended to taper the anti-inflammatory dosage concurrent with the first and third injections of melarsomine. In addition, prednisone administered concurrently with doxycycline is recommended if there are clinical signs of heartworm disease.8
Slow-Kill Methods
Numerous studies have evaluated the efficacy of slow-kill modalities, which consist of a prophylactic dose of ivermectin, topical moxidectin, or injectable moxidectin and concurrent administration of doxycycline as an adulticidal protocol.15-18 These protocols have varying efficacy and require a longer time period than the 3-dose regimen of melarsomine. Although these protocols may be considered advantageous in certain settings, they are salvage procedures. The use of the slow-kill method versus the 3-dose regimen is analogous to femoral head osteotomy versus total hip replacement in a dog. The slow-kill method can be successful, but it is not ideal, as the worms remain in the vessels longer, and the time of worm death is variable as compared with melarsomine treatment.
Pulmonary Thromboembolism & Pulmonary Damage
PTE and pulmonary damage are inevitable consequences of successful adulticidal therapy. Although no current tests are predictive for PTE, the severity of clinical signs can be reduced via administration of doxycycline and corticosteroids during treatment.8 Clinical signs of embolism include fever, cough, hemoptysis, and exacerbation of right-sided heart failure. These signs are usually seen within 7 to 10 days but may occur for up to 4 weeks posttreatment. Therefore, exercise restriction for 6 to 8 weeks posttreatment (total of 10-12 weeks) is essential.8 This can be difficult, but cage rest (leash walk only) is as important as melarsomine injections.
Compromise may be necessary to ensure adherence to exercise restriction. If the dog is anxious in a crate, confinement in a small room may be preferred to ease anxiety. Assessing the ease at which the pet owner is able to restrict the dog can help with tailoring exercise restriction recommendations. For example, the owner may be asked to rate (on a scale of 1 to 10) the ease of crate resting the dog for 10 to 12 weeks (considering this duration includes 4 weeks after the first injection of melarsomine, then 6-8 weeks after the second and third injections of melarsomine). Recommendations can be given based on the answer.
Prognosis & Prevention
Prognosis is typically good with treatment and appropriate exercise restriction. To prevent reinfection, preventives labeled for use against heartworms should be adherently administered.
Clinical Follow-Up/Monitoring
Dogs should be tested for microfilariae 30 days posttreatment. If the test is positive, a microfilaricide should be given. A test for antigen should be given 9 months post-treatment. If the test is positive, the dog should be retreated with a 2-dose protocol.
Conclusion
Treatment recommendations for heartworm disease are constantly changing. It is thus important to keep current on developments. The 3-dose treatment protocol is the recommended approach to treatment in most cases.