Canine Atopic Dermatitis
Maite Verde, DVM, PhD, Zaragoza University
Profile
Canine atopic dermatitis (CAD) is a genetically predisposed inflammatory, pruritic skin disease.
Characteristic signs, associated with immunoglobulin (IgE), are most commonly directed against environmental allergens1; however, CAD can be associated with other systemic signs (eg, GI, respiratory).
Allergens not associated with environment (primarily food) may trigger dermatitis flare-ups with signs indistinguishable from CAD.
Food can induce atopic dermatitis.
Atopic-like dermatitis (ALD) is an inflammatory, pruritic skin disease with clinical features identical to those in CAD, in which an IgE response to environmental or other allergens cannot be documented.2
ALD, similar to intrinsic atopy in humans, describes patients with clinical features of CAD and no detectable IgE increase.2
This definition assumes that IgE is not necessary for clinical manifestations of the disease and that other mechanisms (eg, skin barrier dysfunction) can lead to dermatitis clinically indistinguishable from classic atopic dermatitis.
Pathophysiology
CAD is a complex, multifactorial disease; genetic and environmental factors play a fundamental role.
CAD is triggered mainly by aeroallergens; diverse factors (eg, bacterial or yeast overgrowth, physiologic or weather factors) can affect its presentation.3
The 2 major mechanisms of the disease are:
Abnormalities of the epidermal structure and function.
Cutaneous inflammation due to inappropriate immune response to antigens encountered on the skin.
A defective cutaneous barrier is considered crucial for development of atopic dermatitis.3
In patients with atopic dermatitis (AD), the epidermal elements that should form a compact wall are unstructured; this leaves weak points, which allergens can penetrate.
Defects or dysfunction in structural skin integrity (eg, corneodesmosomes, intercellular lipids, terminally differentiated keratinocytes), are associated with CAD development.4
Cutaneous barrier dysfunction can be caused by5:
Intercellular lipid lamellae structural defects in the stratum corneum and at the junction with the stratum granulosum.
Defects of skin lipid composition with reduced ceramides and reduced expression of and mutations of filaggrin (ie, structural protein isolated from the stratum corneum that binds to keratin filaments and causes aggregation into macrofibrils contributing to cellular compaction and a highly insoluble keratin matrix).
The matrix acts as a protein scaffold for the attachment of cornified-envelope proteins and lipids that form the stratum corneum.6
Aberrant lamellar organization and increased transepidermal water loss.
An overactive T-helper type 2 (Th2) immune response against environmental allergens that penetrate the epidermis can lead to increased production of allergen-specific IgE (not in ALD cases) and to an inflammatory dermal reaction that worsens cutaneous barrier function, appearance of lesions, and pruritus.
Bacteria and yeast (eg, Staphyloccocus pseudintermedius, Malassezia pachydermatis), which easily colonize the skin surface,3 adhere and multiply faster in individuals with AD.
Overgrowth is common and can produce recurrent pyoderma and Malassezia spp dermatitis.
Bacteria and Malassezia spp may also act as antigens producing bacterial and Malassezia spp hypersensitivity, which worsens the inflammatory process.
The enteric barrier and immunologic tolerance mechanisms in the GI tract may present functional defects.
Some patients could develop hypersensitivity to allergens, particularly protein, in the diet.
Factors that can trigger flares or worsen the disease include flea bites, food, inhalant, and contact allergens.7
Some detergents, some textile fibers, extreme temperatures and humidity, and cutaneous microbial colonization can have the same effect.
Related Articles
Signalment
Age of onset can range from 6 months to 6 years,8 depending on factors such as breed and geographical location.
Highly susceptible dogs in warm climates, with pollen present year-round, have increased risk for early onset of signs.8
French bulldogs and shar-peis appear to develop CAD earlier in life than other breeds.9
Dogs with food-induced CAD are more likely to be presented with clinical signs at <1 year of age in 50% of cases; for CAD related to environmental allergens, it is 38%.3
In general, clinical signs appear before 3 years of age in 68% of cases.10,11
Breed predisposition and breed-specific clinical phenotypes have been reported.9,12
Clinical Signs
CAD is a clinical syndrome, not a uniform disease.
Clinical manifestations can evolve throughout the life of affected dogs.
Some dogs are presented with year-round clinical signs from the onset of CAD.
In about 30% of cases, signs are seasonal and associated with environmental allergens.8
In these cases, signs may become present year-round as the disease progresses.
Dogs with food-induced atopic dermatitis or CAD caused by dust mites are presented with year-round clinical signs.8
Pruritus without lesions (ie, sine materia) at onset is the main sign of the disease.
This can be accompanied by erythema and papules as initial lesions in affected areas.
Early in the disease process, pruritic intensity can be mild (eg, 4-5 on a scale of 1-10) but increases progressively as the process becomes chronic and/or is complicated with secondary infections (eg, bacterial or yeast overgrowth) or other aggravating factors (eg, food, fleas, contact irritants).
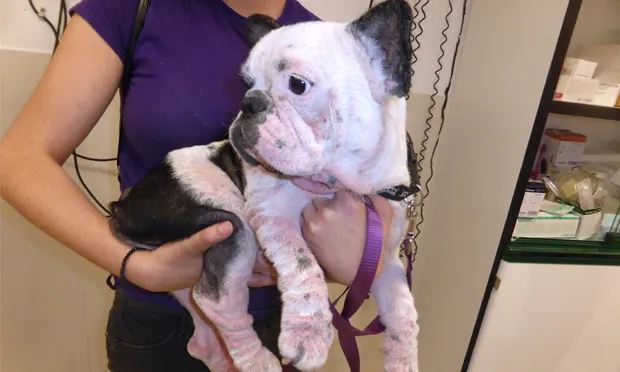
Lesions are not specific, but their distribution pattern can be highly suggestive of CAD (Figure 1).
Lesions observed in the acute phase include erythema and papulopustular rash that evolve to squamous lesions, lichenification, and alopecia as the disease progresses.
Areas most commonly affected are ventral hairless zones (axillae, inguinal region, and interdigital areas; Figure 2), similar to the lesions seen in allergic contact dermatitis.
Other affected areas include the muzzle, periocular region, pinnae (Figure 3), and flexural surface of the elbow (Figure 4).
Other signs may include conjunctivitis, otitis (Figure 5), hyperhidrosis, chronic changes from pruritic behavior (eg, salivary staining, lichenification, hyperpigmentation; Figures 6 and 7), acute moist dermatitis, acral pruritic nodules, and acral lick granulomas.
In up to 43% of cases, external otitis can be the first sign.8
Some dogs with CAD are predisposed:
To develop reactions to allergens (eg, food, flea, contact allergens).
To develop secondary bacterial infections (66%) and yeast infections (33%) with some breed propensity (eg, West Highland white terrier, German shepherd dog).7
Concurrent environmental and food allergens have been observed in 13% to 30% of cases10; in these patients, GI manifestations (eg, increased frequency of defecation, soft and light-colored feces, flatulence, scooting) can accompany the cutaneous signs.
Other noncutaneous signs (eg, rhinitis, reverse sneezing, alteration of the estrus cycle) can be observed.
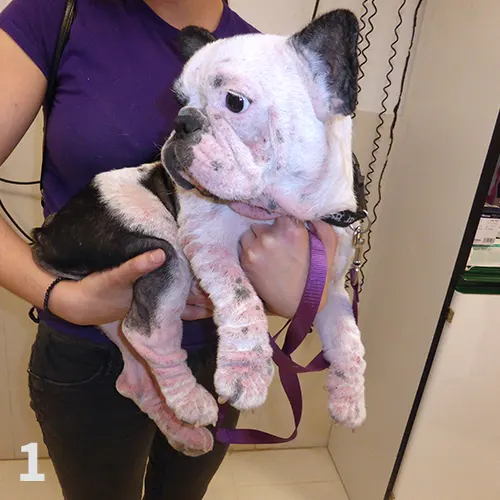
Figure 1 Dog with atopic dermatitis and generalized lesions.
Diagnosis
Based on presentation frequency of different signs, a set of diagnosis criteria has been proposed (see Favrot’s Clinical Critera Sets).12
When 5 of these criteria are present, this provides 85.4% sensitivity and 79.1% specificity for CAD diagnosis.
Favrot’s Clinical Criteria Sets12
All sets of criteria should be used only after ruling out other causes of pruritus (eg, ectoparasites, infections, food).
Set 1 Criteria
Age at onset <3 years
Mostly indoor
Corticosteroid-responsive pruritus
Chronic or recurrent yeast infections
Affected front feet
Affected ear pinnae
Non-affected ear margins
Non-affected dorsolumbar area
Dogs exhibiting 5 of these criteria have 85.4% sensitivity and 79.1% specificity for diagnosis of CAD12
Dogs exhibiting 6 of these criteria have 58.2% sensitivity and 88.5% specificity for diagnosis of CAD12
Set 2 Criteria
Age at onset <3 years
Mostly indoor
Pruritus sine materia (ie, without lesions) at onset
Affected front feet
Affected ear pinnae
Non-affected ear margins
Non-affected dorsolumbar area
Dogs exhibiting 5 of these criteria have 77.2% sensitivity and 83.0% specificity for diagnosis of CAD12
Dogs exhibiting 6 of these criteria have 42.0% sensitivity and 93.7% specificity for diagnosis of CAD12
Diagnosis must be based on clinical history, signs, and exclusion of other pruritic causes of dermatitis with similar clinical presentations.
There is no definitive test for CAD diagnosis.
For a patient with a clinical history and signs suggestive of CAD, these steps can be followed to reach a definitive diagnosis:
A differential diagnosis should be made based on information from clinical history and physical and dermatological examination (Table 1).
Table 1: Canine Atopic Dermatitis Differential Diagnosis: Causes to Be Ruled Out
It should be verified that the patient meets at least 5 of Favrot’s diagnostic criteria.
Basic dermatological diagnostic tests and/or therapeutic trials should be conducted to rule out Sarcoptes scabiei, Demodex spp mite infestation, pyoderma, and yeast overgrowth as causes of pruritus.
Sarcoptic mange should be ruled out.
Superficial scrapings on the periphery of alopecic lesions and squamous scabs; lesions on edge of the pinnae, hocks, and elbows; and evident pinnal-pedal response can help in diagnosing Sarcoptes.
In case of doubt, 4 doses of selamectin or moxidectin spot on should be applied every 2 weeks for 2 months; other options (eg, PO/SC ivermectin, lime sulfur dips) may be considered.
Demodex spp mites can be found in deep skin scrapings of dogs with signs similar to those seen with CAD.
These mites are readily found on deep skin scrapings of alopecic lesions; blood should be visible on cytologic samples.
Demodex spp mites may be noted in dogs previously treated with systemic corticosteroid medications; in this situation, demodicosis may resolve strictly with discontinuation of steroid therapy.
Bacteria overgrowth, surface folliculitis, and pyoderma are common in CAD.
Topical therapy (ie, antiseptic shampoos 2 times a week at onset and later once a week) may be indicated and can resolve pyoderma in some patients (Table 2).
In other cases, treatment with systemic antibiotics may be warranted, although they should be used judiciously to help prevent the development of bacterial resistance.
Yeast overgrowth, common in CAD, can significantly contribute to pruritus.
Malassezia spp can trigger hypersensitivity reactions; if yeast is found on surface cytology, topical therapy (eg, chlorhexidine and miconazole baths) or systemic therapy with antifungals (eg, itraconazole ketoconazole, fluconazole, terbinafine) should be initiated.
Control of internal and external parasites (eg, fleas) should be verified.
Adulticidal flea preventative should be administered consistently to all potentially allergic patients in geographical regions endemic for fleas.
A strict elimination diet can detect whether diet components are responsible for >75% of pruritus and clinical signs.13
In many CAD cases, patients are presented with clinical signs caused by a combination of food and environmental allergens.
In these cases, in the author’s experience, only a mild improvement (<25%) may be observed during the elimination diet.
If no substantial improvement of signs and/or pruritus has been seen following the steps listed above, and if the patient has at least 5 of Favrot’s clinical criteria, the patient can be diagnosed CAD to aeroallergens, with 85.4% sensitivity and 79.1% specificity.
Once diagnosed, the patient can be treated based on signs (Table 2), or an intradermal or serological ELISA allergen test can be performed to identify environmental allergens involved in development of clinical signs.
If positive results are obtained in the intradermal or serologic test, and these are in line with the epidemiological characteristics and the patient history, immunotherapy may be recommended.
Table 2: Canine Atopic Dermatitis Therapy
Systemic (PO) Administration
*Gloves should be worn.
Treatment
Treatment must be adapted to each patient; there is no set formula for CAD treatment.
The right therapeutic approach for each patient will be based on concomitant factors (eg, geographical area, severity of clinical signs, duration of signs, acute or chronic presentation, patient age, owner resources).
Pruritus threshold and summary effects are critical in managing CAD.
All pruritus-inducing factors should be analyzed in determining which are most important to control or eliminate.
Client Communication
Clients should be informed that:
CAD is a chronic, incurable disease.
Pruritus may not resolve completely; however, with therapy it should markedly improve to a reasonably tolerable level.
The veterinary team will try to use as few drugs as possible to minimize or control signs.
The patient can relapse, and the condition can worsen with age.
It is important to be strict with diet and flea control.
Measures to lower the concentration of aeroallergens may help prevent relapses.
Minimizing Allergen Exposure
Allergen exposure can be minimized by:
Giving baths to reduce epicutaneous exposure.
Preventing atopic dogs from walking on grass, particularly recently cut grass.
Eliminating carpets and rugs from the house.
Frequently vacuuming curtains and fabric-covered furniture.
Frequently laundering the dog bed with hot water to reduce mites.
Keeping the home free of tobacco smoke.
Controlling the relative humidity and temperature using air conditioning.
Skin Barrier Repair
The following methods can repair the skin barrier:
Frequent baths (2 times a week initially, then less frequently) with products containing antiseptics, antifungals, emollients, and moisturizers can help control bacterial and/or Malassezia spp overgrowth, folliculitis, and superficial pyodermas.
Baths also have a hydrating effect and can wash allergens off of the skin surface.
Systemic antibiotics for at least 4 weeks (in superficial pyodermas) or 8 weeks (in deep pyodermas) if pyoderma cannot be controlled with topical treatment.
Systemic antifungals in case of Malassezia spp overgrowth and the associated signs are significant and not controlled with topical treatment.
Antiparasitic Control
Use GI parasite and flea control year-round.
Adjust the preventive program to fit the area’s climate, the patient’s living environment (eg, indoor, outdoor, additional pets), and bathing frequency.
Diet
In CAD cases in which a dietary component is suggested, patients should be fed a hypoallergenic diet with specific or hydrolyzed proteins.
In cases that are not food-induced, diet should be supplemented with essential fatty acids.
Pruritus Therapy
These drugs may control pruritus and inflammatory lesions that could not be resolved with therapies described previously14:
H1 antihistamines with essential fatty acids.
Oclacitinib at 0.5 mg/kg twice a day for 1 to 2 weeks, then lower the dose and frequency to control acute crises and for mid- and long-term therapy.
Corticosteroids (prednisolone and methylprednisolone) at 0.5 mg/kg twice a day for 1 week, then lower to once a day and once every other day to treat acute crises.
Modified cyclosporine A at 5 mg/kg once a day for a month, then lower the frequency, for long-term treatment.
Modified cyclosporine A can be combined with oclacitinib and corticosteroids for the first 2 weeks of treatment.
Topical therapy with corticosteroids and tacrolimus in focal-localized cases.
Immunotherapy
Allergen-specific immunotherapy (ASIT) or hyposensitization is considered the only treatment that could alter the course of the disease.
ASIT may be a good option for long-term control of CAD, but it is not effective for every patient.
ASIT allows for modulation of the immune system through administration (SC or sublingual) of allergen concentrates to which the patient has shown to be sensitive (based on the results of allergen testing), with increasing doses and frequencies.
Immunotherapy is the best option, particularly with young patients with nonseasonal CAD and in patients that are expected to stay in the same area in the future.
In the author’s experience, once good results are achieved, hyposensitization therapy should be maintained for life.
Not every CAD patient will benefit from immunotherapy, which has been shown effective in approximately 50% to 75% of cases.15
However, when a formulation has been unsuccessful (eg, a patient has not benefited from injectable immunotherapy), another (eg, sublingual administration) may have beneficial results.
Monoclonal antibody therapy for the immediate future.16
Proposed to inhibit IgE production via its promoting cytokines (IL-4/ IL-13), their cytokine receptors, or IgE itself.
The itch sensation itself could be altered, at least theoretically, by antibodies targeting itch-promoting cytokines such as IL-31.
The newly available monoclonal antibody product against IL-31 can be administered SC once-monthly.
Safety and efficacy studies are still ongoing with the conditional license.