Bronchoalveolar Lavage Fluid Collection Using a Blind Technique
Carol R. Reinero, DVM, PhD, DACVIM (SAIM), University of Missouri
Disorders of the respiratory tract in the dog and cat are common and include infectious, noninfectious, inflammatory/fibrotic, and neoplastic diseases. Microscopic evaluation and culture of cells obtained from the airways and pulmonary parenchyma can help definitively identify a variety of infectious organisms and neoplastic cells.
Cytologic specimens may be obtained by trans- or endotracheal lavage, bronchoalveolar lavage (BAL), bronchial brushings, or transthoracic fine needle aspiration. BAL is an excellent way to obtain cellular and acellular samples from the lower airways, interstitium, and alveoli, and has the advantage of recovering specimens representative of comparatively large regions of the lung.
Techniques
While use of a bronchoscope is the standard method for accessing a small airway to obtain fluid for cytology and culture, bronchoscopes are expensive, require technical expertise, and are not available in every practice. However, in small dogs and cats, a blind technique for collecting BAL fluid can be performed quickly, easily, and without specialized equipment. This procedure is similar to an endotracheal wash, except the tube used is placed further down the respiratory tract. The presence of foamy surfactant indicates a deep lavage of the lung and, along with the distance the tube is inserted, helps differentiate this procedure from an endotracheal wash.
Related Reading: Top 5 Respiratory Diagnostics
Step-by-Step: Collecting Bronchoalveolar Lavage Fluid Using a Blind Technique
What You Will Need
Laryngoscope with an appropriately sized blade
Lidocaine
Sterile endotracheal tube
Sterile red rubber or polypropylene catheter
Sterile 0.9% saline
Multiple 20-mL syringes
Two sterile tubes without additives (1 for cytology, 1 for culture)

Most cats and small dogs will use an endotracheal tube size ≥4; an 8-French red rubber or polypropylene catheter will fit through the lumen of these tubes. It is recommended that in sterile fashion, the distal tip of the catheter be cut off and smoothed out so that sterile saline will easily flow out the end instead of side holes. Generally, use of sharp, sterile scissors is adequate to make a smooth cut; however, for polypropylene catheters, using a sterile metal file may also ensure the edges are not too sharp. Additionally, the proximal end will need to be trimmed so that a 20-mL syringe will snugly attach.
Step 1
A short-acting anesthetic (eg, propofol, ketamine, valium, etc) is administered intravenously. In experienced hands, the entire procedure takes only 1 to 2 minutes. Topical lidocaine is placed on the arytenoids to prevent laryngospasm, and the sterile endotracheal tube inserted, taking care to avoid contaminating the tip of the endotracheal tube in the oral cavity.

Step 2
Place the animal in lateral recumbency with the neck outstretched. Providing supplemental oxygen prior to and following the BAL fluid collection is recommended.

Step 3
The sterile red rubber or polypropylene catheter is inserted into and gently advanced through the lumen of the endotracheal tube until firm resistance is felt (corresponding to when it is lodged in a small airway). Care must be taken not to force the catheter too far in the airway, especially a rigid polypropylene catheter, or an iatrogenic pneumothorax may be induced. However, if a good wedge is not achieved, the yield may be poor.
Expert Insight
Measure the catheter against the endotracheal tube prior to beginning the procedure to make sure the catheter advances past the end of the tube during insertion.
Consider administering an injectable bronchodilator (eg, terbutaline, aminophylline) to minimize bronchospasm prior to performing this procedure in cats, especially those with suspected lower airway disease (eg, feline asthma).

Step 4
A syringe filled with warmed sterile saline is attached to the proximal end of the polypropylene catheter, and a 10- to 25-mL aliquot is steadily and rapidly instilled. In dogs, the procedure is often repeated using a total final volume of instilled saline of 50 to 75 mL; in cats, up to 60 mL has been described. However, it is important to monitor the clinical status of the patient to judge if repeating the lavage is advisable.
In my experience, it is generally not necessary to instill more than 1 aliquot in a cat and 1 to 2 aliquots in a dog. If there is a poor yield of fluid retrieved (< 30%) or if the animal is showing evidence of respiratory compromise, it may be prudent to stop with a smaller total volume of instilled fluid. Since the lumen of the endotracheal tube is in large part obstructed by the catheter, the instillation and aspiration of saline must be done efficiently.

Step 5
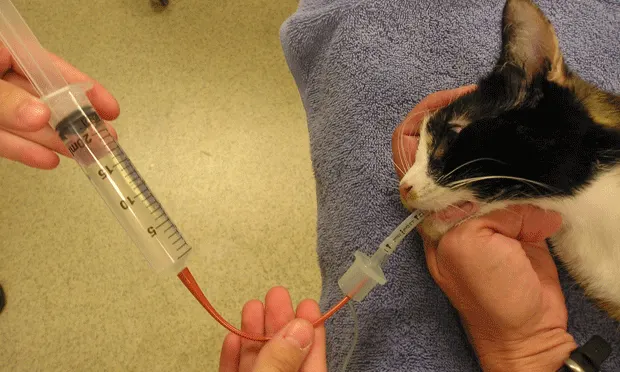
Using gentle hand suction, the fluid is aspirated back out of the airways. As negative pressure in the syringe is felt, the pressure is let off the syringe, the catheter just slightly backed out, and aspiration is continued (A). As needed, the hindquarters of the animal may be elevated during the aspiration to allow gravity to assist with fluid retrieval (B).
The presence of foam in the retrieved fluid indicates surfactant and a deep alveolar lavage. The volume of saline retrieved ranges from roughly 30% to 80% of the amount instilled. If an inadequate volume is retrieved or if surfactant is not seen, the procedure should be repeated. When finished, the aliquots of BAL fluid may be pooled and then divided into 2 storage tubes, one for cytology and the other for culture (C).



Step 6
After the saline has been retrieved, the catheter should be completely removed from the endotracheal tube. The hindquarters of the animal may again be elevated while an assistant performs a short period of coupage to allow for drainage of fluid remaining in the larger airways. Recovery should be assisted by supplemental oxygen, and monitoring in the short term is recommended with a pulse oximeter. Animals should be monitored for cyanosis or respiratory distress for 10 to 15 minutes after the procedure.
