Bacterial Biofilms
Meagan Walker, BSc, University of Guelph
Ameet Singh, DVM, DVSc, DACVS (Small Animal), University of Guelph
J. Scott Weese, DVM, DVSc, DACVIM, FCAHS, University of Guelph, Ontario, Canada
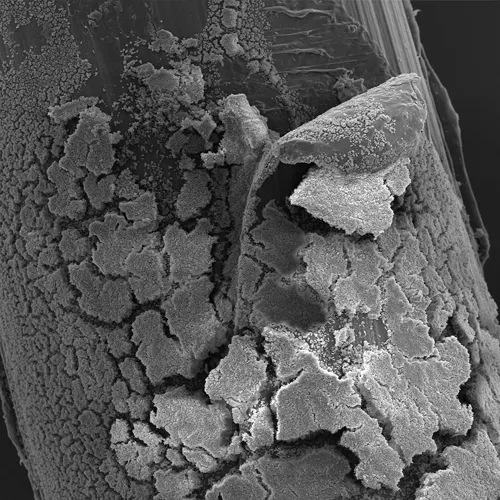
Scanning electron micrograph of a bacterial biofilm on an orthopedic screw.
What are bacterial biofilms, and why are they important in small animal practice?
A bacterial biofilm is a complex community of bacteria embedded within a self-produced matrix (ie, slime).1,2 An example of a bacterial biofilm is the slimy surface that accumulates in water bowls or the plaque that forms on teeth. In natural environments, bacteria exist in 2 states: the planktonic (ie, free-floating or nonbiofilm-embedded) state or the biofilm-embedded (ie, adhered to a surface) state.2 The planktonic state is important in the replication and growth of bacteria; however, bacteria have a tendency to congregate together and adhere to a surface.2 The biofilm-embedded state enables this congregation and adherence, which also allows for protection from harsh environmental conditions.2 Bacterial biofilms have been associated with persistent surgical site, wound, and urinary tract infections.2
Biofilm formation is a complex process during which bacteria adhere to a noninert (ie, living [eg, GI tract, teeth, gums]) or inert surface (eg, surgical implant, catheter, suture), grow, and produce a film-like matrix to protect themselves from the host immune response and antimicrobial therapy.2,3 For reasons yet to be determined, when bacteria detach from the biofilm, planktonic bacteria are released from the biofilm, which enables dissemination of the infection and potentially leads to biofilm formation and reattachment at other distant sites.2,3 The detachment phase may occur when the biofilm’s nutrient resources have been depleted.1,2 The detachment phase may occur days, weeks, or even years after initial biofilm formation and can result in clinical signs of planktonic infection.1,2 For example, the authors have observed implant-associated infections several years after surgery and have speculated that this is a result of the detachment of planktonic bacteria from a biofilm associated with the surgical implant.
Common causes of biofilm-associated infection include orthopedic implant infections (often caused by Staphylococcus pseudintermedius and other gram-positive pathogens), urinary tract and catheter-associated infections (eg, Escherichia coli), dental plaque formation and gingivitis (eg, Neisseria spp), and otitis (eg, Pseudomonas aeruginosa).3
Related Article: Methicillin-Resistant Staphylococcus pseudintermedius Biofilm Formation & Antimicrobial Susceptibility
Why Are Biofilms Important to Clinicians?
Surgical site, wound, and urinary tract infections are rapidly emerging problems in veterinary medicine. These infections are often associated with patient morbidity and mortality, increased treatment costs, prolonged hospitalization, and owner and veterinarian frustration.4 Surgical site infections may account for up to 25% of hospital-associated infections,5 and several studies have reported surgical site infection rates that range from 0.8% to 21.3% of surgical cases.6-10 A recent study in dogs showed the economic impact of surgical site infections after tibial-plateau–leveling osteotomy surgery to be $110.21 to $3817.12 USD.8 Orthopedic implant infections associated with a biofilm can be difficult to treat because biofilms inhibit penetration of antimicrobials and cells of the immune system.2,3,11 In many cases of biofilm-associated implant infections, implant removal is often the only choice for eliminating the biofilm.4,8 Antimicrobial therapy is often administered to treat clinical signs associated with the infection until the implant can be removed (eg, fracture consolidation following orthopedic implant placement).9
In vitro techniques in many bacteria (eg, S aureus, S pseudintermedius, P aeruginosa, E coli, Trueperella pyogenes, Cornyebacterium renale) have demonstrated the ability of these bacteria to produce a bacterial biofilm.12-14 A study in dogs and cats evaluating the biofilm-forming ability of S pseudintermedius, the most common pathogen isolated from canine and feline surgical site infections, classified 96% of isolates as strong-to-moderate biofilm producers.10 These results highlight the importance of biofilm prevention vs treatment.
Does My Patient Have a Biofilm Infection?
There is no specific test to determine whether an infection is associated with a biofilm,13 and it may be difficult to identify an infectious cause when a biofilm is involved in persistent wounds or surgical site infections. Biofilm-associated bacteria may not be readily accessible for samples obtained using standard culture swab techniques, and false-negative cultures are not uncommon, as a standard swab may not pick up bacteria in a biofilm. Furthermore, even if bacterial samples are obtained, bacterial metabolic rates drastically reduce in a biofilm as a measure to reduce nutrient consumption, which also reduces replication rates and makes growth using standard agar media challenging.4 This may occur particularly with implant-associated infection.4
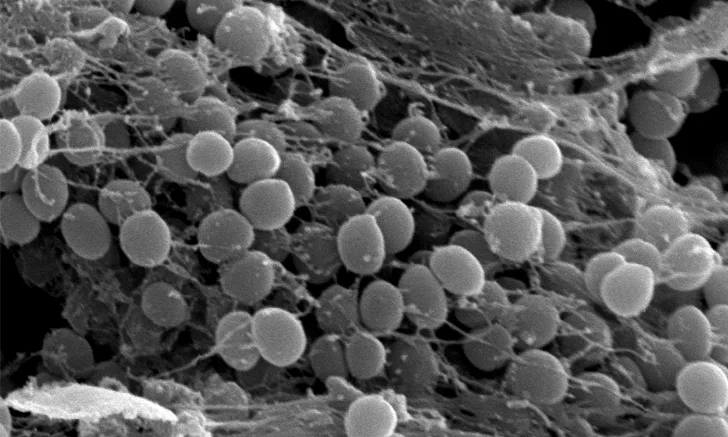
Scanning electron micrograph of a Staphylococcus pseudintermedius biofilm adhered to barbed monofilament polydioxanone suture. The biofilm is seen adherent to the suture, particularly around the barb.
How Do I Determine if an Infection Involves a Biofilm?
These guidelines can help in the diagnosis of biofilm infections.4
Is there a medical history of implants in that location being associated with biofilm infections (eg, orthopedic implants, indwelling catheters, pacemakers)?
Has the patient had a recurrence of the infection, particularly of the same organism being identified at various time points?
Has the patient experienced antibiotic failure or persistent infection despite appropriate therapy selection based on in vitro culture and susceptibility testing?
Is there evidence of local or systemic signs that resolve with antibiotic therapy but recur after therapy completion?
Was the infection potentially caused by an invasive device (eg, catheter) or implant?
How Do I Treat a Biofilm Infection?
Bacterial culture and antimicrobial susceptibility testing are important for confirming a bacterial infection, identifying the responsible organism, and directing antimicrobial therapy; however, testing has limitations.14 Antimicrobial efficacy is traditionally evaluated by determining the minimum inhibitory concentration (MIC; ie, the lowest concentration of an antimicrobial able to inhibit visible bacterial growth).13 Most MIC-determining techniques test only the planktonic bacteria and not the biofilm-embedded bacteria.13 For some antimicrobials, the concentration required to inhibit bacterial growth in a biofilm can be more than 1000 times greater than that required to kill planktonic bacteria of the same strain13-16; therefore, antimicrobials chosen based on standard methods of bacterial culture and susceptibility testing may prove ineffective, as the results do not necessarily indicate susceptibility of bacteria in a biofilm.
Treatment options for biofilm infections are limited. Antimicrobial therapy may resolve clinical signs of a planktonic infection; however, clinical signs often recur after treatment stops.4
Infections involving an orthopedic implant are especially difficult to treat because of bacteria adherence to the implant. Debridement of infected tissue and removal of the implant following bone union is the recommended treatment for both human and veterinary patients.17 Fractures can heal in the presence of a biofilm infection if there is sufficient stability; however, delayed healing can occur.4 Removal of the implant, and therefore the biofilm, is often needed to effectively resolve the infection.17 Treatment options until the implant can be removed may include prolonged antimicrobial treatment, local antimicrobial therapy, and combination antimicrobial therapy.17
With persistent soft-tissue wound infections, the treatment of choice is debridement followed by topical/local antimicrobial therapy, +/- systemic antimicrobial therapy based on culture results.18 Primary wound closure should not be performed until a healthy bed of granulation tissue has formed.
How Can I Prevent Biofilm Infections?
Because of the difficulties inherent in the treatment of biofilm-associated infections, prevention of infections is critical—particularly prevention of infection in cases in which biofilm is more likely to be produced (eg, orthopedic surgery with implants). Strict aseptic technique, hand hygiene, and appropriate selection and use of prophylactic and postoperative antimicrobials are standard in preventing biofilm-associated surgical site infections.4 Avoiding the use of indwelling urinary catheters when possible, promoting hand hygiene, and avoiding manipulation and contamination of catheters should be practiced to reduce risk for catheter-associated infections.4
With the increasing incidence of multidrug-resistant and biofilm-associated infections, there is increased interest in local therapies and techniques as a means of biofilm prevention.19 Coating implants with antimicrobial agents, biocides (eg, chlorhexidine), and/or ion coatings (eg, silver, zinc) may interrupt the biofilm attachment phase and prevent biofilm formation, although veterinary studies of this are lacking.
Future Directions for the Treatment of Biofilm Infections
Due to the prominence of biofilms in human and veterinary medicine, a number of different approaches are being investigated. This includes measures such as development of substances that disrupt biofilm,17 validation of methods to accurately determine antimicrobial susceptibility of biofilm-embedded bacteria,13 use of novel materials and material coating (eg, titanium and nanoparticle silver ion coating) to reduce biofilm formation on implants,20 and use of drugs that downregulate or inhibit biofilm formation.21 Although efficacy data for treatment for or prevention of infections in veterinary patients is currently lacking, a combination of approaches may be available in the near future to help manage these challenging infections.
MIC = minimum inhibitory concentration