Bacteria & Corneal Stains
Audrey Pierson, DVM, DACVO, Gulf Coast Veterinary Specialists, Houston, Texas
In the Literature
Ferreira TAC, Warth JFG, dos Santos LL, Moore BA, Montiani-Ferreira F. Antimicrobial activity of topical dyes used in clinical veterinary ophthalmology. Vet Ophthalmol. 2020;23(3):497-505.
The Research …
Corneal integrity is necessary for clear, functional vision. Although the cornea’s natural lack of vascularization and immune privilege supports corneal clarity, defects in the corneal surface can predispose it to vision-threatening infections. Commonly used corneal stains help assess the integrity of the corneal surface. This study sought to evaluate how these corneal stains affect assessment of corneal infection.
Three basic ocular surface stains (ie, fluorescein, rose bengal, lissamine green) were assessed. Fluorescein is hydrophilic and adheres to intercellular spaces and stromal connective tissue. This stain is used most commonly to screen for corneal ulcerations; it is also used to determine nasolacrimal transit time and tear film stability. Rose bengal stain is used to identify tear film abnormalities and superficial corneal erosions; both degenerate and normal cells stain in the presence of an abnormal tear film with rose bengal only. Lissamine green, although similar in staining pattern to rose bengal, does not stain healthy cells, regardless of tear film dynamics.
In the first part of this study, the impact of these stains on the growth of gram-positive and gram-negative bacteria commonly encountered with ocular surface infections was evaluated. Through the Kirby-Bauer disk-diffusion method, strips containing 3 different amounts (0.01, 0.1, and 1.0 mg) of each stain were applied to plates containing a pure culture inoculum of each bacterial strain being evaluated. The plates were incubated and zones of inhibition were measured. All 3 stains were shown to have antimicrobial activity against the gram-positive bacteria (ie, Staphylococcus aureus, S pseudintermedius, Streptococcus spp); gram-negative bacteria (ie, Escherichia coli, Pseudomonas aeruginosa) exhibited no growth inhibition at lower concentrations of stain and minimal inhibition (ie, resistance) at higher concentrations.
The second part of the study evaluated the effect of the stains on bacteria growth using both preservative-containing and preservative-free formulations of the stains and inoculating them directly with the same bacteria. The presence of bacteria was evaluated over 28 days. The preservative-containing solutions all showed a significant decrease in bacterial counts. All preservative-free stains had some bacteria present after 7 days; at 28 days postinoculation, only preservative-free fluorescein continued to maintain gram-negative bacteria cell counts.

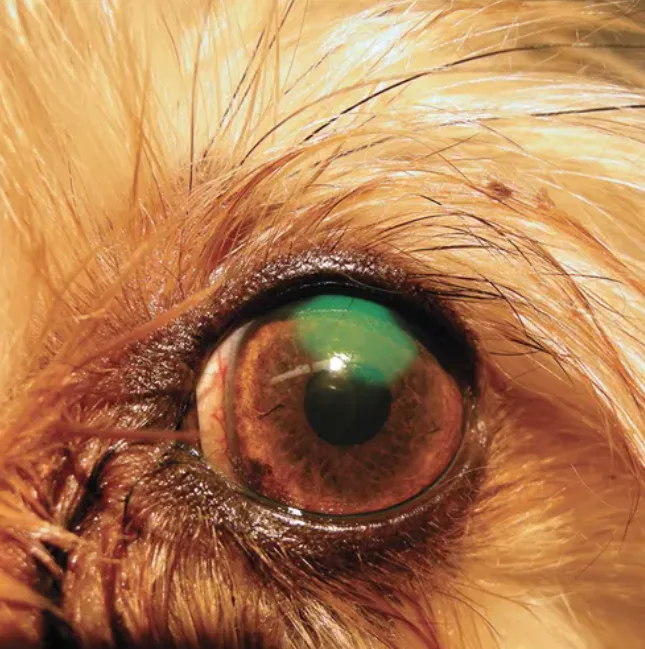
Fluorescein stain applied to the ocular surface of an infected cornea. The diffuse pattern of stain uptake is common in melting corneal ulcers, which may affect culture and susceptibility results.
… The Takeaways
Key pearls to put into practice:
Bacterial culture and susceptibility testing should be performed prior to corneal staining or after copious flushing of the ocular surface after staining.
Preservative-free staining solutions prepared in-house to be used as multidose applications (eg, a fluorescein strip diluted in sterile saline in a syringe) may harbor bacterial colonies; thus, their use is discouraged.
Preservative-containing, commercially available stain preparations appear to prevent bacterial growth and can likely be used for ≥28 days.
You are reading 2-Minute Takeaways, a research summary resource presented by Clinician’s Brief. Clinician’s Brief does not conduct primary research.