Atopica®... Still a Good Choice in the Management of Canine Atopic Dermatitis
Andrew Rosenberg, DVM, DACVD, Animal Dermatology & Allergy Specialists, White Plains, New York, and Riverdale, New Jersey
Brought to you by Elanco Animal Health
Key Points
The safety profile of agents used in CAD therapy is critical because of the life-long nature of the disease.
Cyclosporine has both anti-inflammatory and antipruritic effects and is safe for long-term use. Newer options (e.g., oclacitinib, lokivetmab) can provide short-term relief.
Allergen-specific immunotherapy (ASIT) is the only treatment that can modify or reverse CAD pathogenesis.
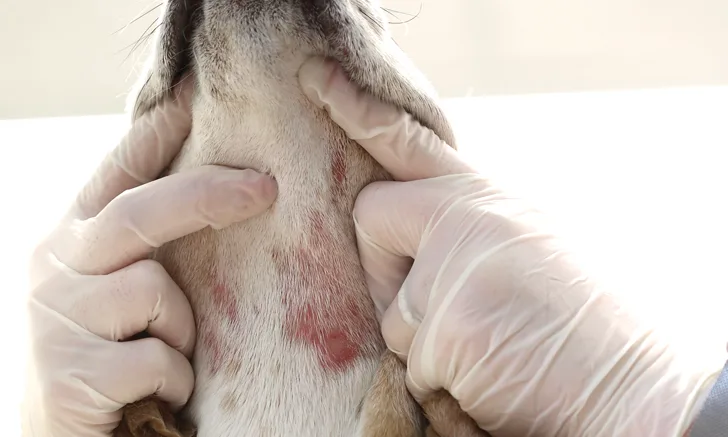
Canine atopic dermatitis (CAD) is one of the most common reasons owners bring their dogs to a veterinarian. This condition is defined as a genetically predisposed inflammatory and pruritic skin disease with characteristic clinical features typically associated with immunoglobulin E (IgE) antibodies directed against environmental allergens.1 CAD is considered a diagnosis of exclusion.
The initial step in the pathogenesis is a defective epidermal barrier that facilitates penetration of potential allergens and microbes. This is followed by a cutaneous inflammatory response characterized by erythema and pruritus. Additional secondary clinical signs include self-excoriation with subsequent secondary superficial pyoderma, Malassezia dermatitis, and/or otitis externa. Treatment goals should include reducing pruritus and inflammation, addressing the defective epidermal barrier, and modulating the abnormal immune response.
Pruritus Relief
Cyclosporine is a safe and effective medication for the long-term treatment of canine atopic dermatitis. It is also helpful at reducing proliferative tissue and inflammation in ear disease associated with atopic dermatitis. The main mechanism of action is calcineurin inhibition, which results in inhibition of IL-2 as well as T-cell activation. As a result, cyclosporine has anti-inflammatory effects as well as antipruritic effects. Cyclosporine has been used by veterinarians for over 20 years and has been licensed (Atopica® [cyclosporine capsules] USP Modified [Elanco]) for the treatment of CAD for over 10 years. In that time, it has shown wide, reproducible evidence of both its efficacy and its safety. A review of 727 dogs treated with cyclosporine showed that approximately one- to two-thirds of patients had a decrease in pruritus of 50% or higher in 4 to 8 weeks.2
With cost of treatment a frequent client concern, two properties of cyclosporine merit consideration. First, the dosing frequency can be tapered. Second, cyclosporine is primarily metabolized by CYP3A enzymes, drugs that inhibit CYP3A (e.g., ketoconazole and other azole antifungals) producing dose-sparing effects when used concurrently. This can potentially reduce the dosage of cyclosporine required.3 The side effects of Atopica are well-known and generally resolve spontaneously or following dose modification or cessation of therapy. Gastrointestinal effects are the most common, typically reported in the first month of therapy. Administering capsules frozen can minimize these effects without affecting pharmacokinetics.4,5
While cyclosporine is effective at reducing pruritus in the long-term, it can take 2-4 weeks of therapy before a clinical benefit is seen. Additional therapeutic options for more immediate pruritus relief include glucocorticoids or newer alternatives such as lokivetmab and oclacitinib. The latter two drugs primarily target the pruritic cytokine IL-31 and are fast-acting. It should be noted that the relatively recent availability of these products limits our understanding of their potential side effects.
Antipuritic Agents for Use in CAD
See Important Safety Information below.
Therapies to Address Barrier Function and Infection
Defective epidermal barrier function should be addressed with topical therapies that can hydrate the skin, reduce allergen penetration, and decrease colonization by microorganisms. Bathing can remove irritants, decrease allergen load and pruritus, and both treat and help mitigate secondary infections. Effective shampoo ingredients include emollients, humectants, ceramides, and moisturizers. “Spot-on” products may help repair the epidermal barrier.
Secondary infections should be addressed or poor overall response is likely. Superior antibacterial efficacy makes chlorhexidine products excellent for CAD, with efficacy against Staphylococcus, Pseudomonas, and Malassezia spp.6 Mild infections can often be managed with topical therapies such as chlorhexidine-based shampoos, sprays, or mousses. In moderate to severe infections, systemic antimicrobial therapies are warranted. Ideally, therapy is based on cytologic evaluation through impression smears and/or cultures when warranted.
Immunotherapy
Allergen-specific immunotherapy (ASIT) is the only treatment that can modify or reverse CAD pathogenesis. Up to 80% of patients have good to excellent response, but clinical benefit can take 4 to 12 months.7 Other therapies are ideally used until ASIT becomes effective and then tapered to the lowest effective dose or discontinued. In some patients, Atopica can be tapered to every other day or less.
INDICATIONS
Atopica® (cyclosporine capsules) USP MODIFIED is indicated for the control of atopic dermatitis in dogs weighing at least 4 pounds (1.8 kg) body weight.
Important Safety Information For Dogs—Atopica
® (cyclosporine capsules) USP MODIFIED: For use only in dogs. Keep this and all drugs out of reach of children. Capsules should not be broken or opened. Wear gloves during administration and wash hands after. Do not use Atopica in dogs with a history of neoplasia, reproducing dogs, or dogs with a hypersensitivity to cyclosporine. Atopica is a systemic immunosuppressant that may increase the susceptibility to infection and the development of neoplasia. Gastrointestinal problems and gingival hyperplasia may occur at the initial recommended dose. Safety and effectiveness have not been established in dogs less than 6 months or 4 lbs. Use with caution in dogs with diabetes mellitus or renal insufficiency, and with drugs that affect the P-450 enzyme system. The most common adverse events are vomiting and diarrhea. Please see product insert for full prescribing information.
Other company and product names are trademarks of their respective owners.
Atopica, Elanco and the diagonal bar logo are trademarks of Eli Lilly and Company or its affiliates.
© 2017 Eli Lilly and Company or its affiliates.
USCACATO00069