Alternative Anticonvulsants for Dogs & Cats
John H. Rossmeisl, Jr., DVM, MS, DACVIM, Virginia Tech

Epilepsy is a commonly encountered condition in small animal practice. Despite the demonstrated efficacy of traditional anticonvulsant drugs such as phenobarbital and bromide, a significant proportion of canine and feline epileptics experience inadequate seizure control or intolerable adverse effects associated with these drugs.1-4
Alternative anticonvulsant drugs (AACDs) such as felbamate,5 gabapentin,4 levetiracetam,3,6 pregabalin,7 topiramate, and zonisamide1 have shown promise for the management of veterinary seizure disorders, and their use is becoming more widespread in veterinary neurology.
With an understanding of the pharmacology and practical considerations of AACDs, clinicians can effectively incorporate these drugs into routine practice, leading to improved seizure control and better quality of life for epileptic pets and their owners.
Traditional anticonvulsant drug doses can often be decreased or even discontinued in pharmacoresistant epileptic patients that have a good to excellent response to AACDs.
Indications
Pharmacoresistant Epilepsy
Pharmacoresistant epilepsy is inadequate seizure control despite documentation of therapeutic steady-state serum concentrations of one or more traditional anticonvulsants, such as phenobarbital or bromide.6 This is the most common indication for adjunctive use of AACDs.1-7
Idiopathic or Secondary Epilepsy
Any of the AACDs described here could be used as a primary monotherapy for dogs or cats with idiopathic or secondary epilepsy. AACDs should be considered as a primary therapeutic option for:
Owners who have concerns about the common adverse effects associated with phenobarbital or bromide treatment for canine or feline epileptics
Dogs and cats with secondary epilepsy resulting from malformation (eg, hydrocephalus), meningoenceph-alitis, or a brain tumor that may have a compromised level of consciousness or other neurologic dysfunction as a result of their disease (AACDs will not result in further sedation, a common effect of phenobarbital and bromide)
Epileptic dogs or cats that have coincidental disease or are receiving concurrent drugs with known or potential interactions with phenobarbital or bromide
Pharmacointolerant Epilepsy
Pharmacointolerant epilepsy is considered to be present in animals that are on phenobarbital and/or bromide and experience clinically intolerable adverse effects, such as sedation, ataxia, polyphagia, polyuria, or polydipsia, at serum concentrations necessary to achieve adequate seizure control.
Although any AACD could be used in cases of pharmacointolerant epilepsy, levetiracetam is a popular choice in this clinical situation because it is rarely associated with adverse effects and rapidly confers anticonvulsant activity.3,6 This allows an immediate decrease in the dose of the problematic traditional anticonvulsant while minimizing the risk for breakthrough seizures.
Contraindications
Felbamate is contraindicated in myelosuppressed patients.5 The proprietary liquid formulation of gabapentin (Neurontin, pfizer.com) is also contraindicated for dogs because it contains xylitol. Zonisamide should be avoided in animals with a history of or predisposition to sulfonamide sensitivity.1 All AACDs should be used with caution and at appropriately modified doses in animals with significant hepatic or renal dysfunction.
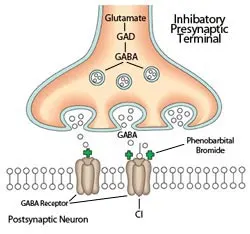
Schematic of inhibitory synapse in the brain. The predominant mechanism of action of barbiturate and benzodiazepine anticonvulsants occurs via postsynaptic facilitation (+) of the GABAA receptor, which results in hyperpolarization of the postsynaptic neuron. Select AACDs may also have direct or indirect GABA-agonist actions (see Table). Illustration by Terry Lawrence
Figure 1. Schematic of inhibitory synapse in the brain. The predominant mechanism of action of barbiturate and benzodiazepine anticonvulsants occurs via postsynaptic facilitation (+) of the GABAA receptor, which results in hyperpolarization of the postsynaptic neuron. Select AACDs may also have direct or indirect GABA-agonist actions (see Table). Illustration by Terry Lawrence
Advantages
The major advantage of AACDs lies in their very high therapeutic index. Clinically, this equates to being able to achieve the desired therapeutic effect (ie, improved seizure control) in a majority of patients with no (or transient and mild) adverse effects. This is particularly advantageous in patients with secondary epilepsy associated with interictal neurologic dysfunction and those with pharmacointolerant epilepsy.
AACDs also provide the pharmacoresistant epileptic patient with an expanded mechanistic approach to seizure suppression (Figures 1 and 2, Table) that may be synergistic with traditional anticonvulsant drugs. Phenobarbital and bromide both work primarily via postsynaptic g-aminobutyric acid (GABA) agonism (Figure 1), as do some AACDs reviewed here.
However, all AACDs also suppress seizures via alternative pathways (Figure 2, Table) and thus theoretically provide broader-spectrum seizure control in patients with epilepsy that is refractory to traditional drugs. Pharmacoresistant epileptic patients that have a good to excellent response to AACDs can often have traditional anticonvulsant drug doses decreased or even discontinued.1,4,6
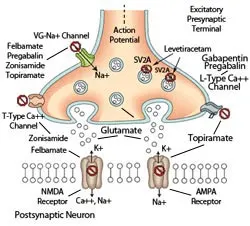
Schematic of excitatory synapse in the brain. AACDs exert their anticonvulsant effects through a variety of mechanisms that inhibit ( ⃠ ) the release of excitatory neurotransmitters, such as glutamate, into the synaptic cleft or by antagonizing ( ⃠ ) the signal transduction that would occur following ligand binding to postsynaptic glutamate receptors (see Table). Illustration by Terry Lawrence
Figure 2. Schematic of excitatory synapse in the brain. AACDs exert their anticonvulsant effects through a variety of mechanisms that inhibit ( ⃠ ) the release of excitatory neurotransmitters, such as glutamate, into the synaptic cleft or by antagonizing ( ⃠ ) the signal transduction that would occur following ligand binding to postsynaptic glutamate receptors (see Table). Illustration by Terry Lawrence
*Extrapolated from therapeutic reference intervals in humans.
Disadvantages
Compared with phenobarbital and bromide, a principal disadvantage of AACDs is that the therapeutic response of an individual to any given drug is much less predictable, especially in cats. For example, appropriate treatment with phenobarbital or bromide monotherapy has reportedly resulted in adequate seizure control in approximately 75% of dogs with idiopathic epilepsy.1 There is currently insufficient published information regarding the therapeutic efficacy of AACD monotherapy for idiopathic epilepsy to be able to make similar predictions.
Other potential disadvantages include dosing and cost. Several AACDs require Q 8 H administration to achieve therapeutic serum concentrations, and all AACDs described here are more expensive than traditional anticonvulsants.
Although not a disadvantage of AACDs, a major obstacle when managing any epileptic pet is the significant commitment required of the client to adhere to home care guidelines, routine professional recheck evaluations, and therapeutic monitoring recommendations. Therapeutic failures should be anticipated, regardless of the anticonvulsant(s) used, unless the client is properly educated about—and willing to make—the commitment necessary to manage epilepsy.
Economic Impact
Although daily drug costs associated with AACD administration are higher than those for phenobarbital or bromide, generic formulations of most AACDs are available and economical. For example, a 30-kg dog with epilepsy can be treated with currently available generic formulations of gabapentin, levetiracetam, topiramate, or zonisamide for $25 to $80 per month. Both felbamate and pregabalin are typically cost-prohibitive for use in large- or giant-breed dogs.
Clients should be advised that the up-front investment in AACDs can greatly improve the pet’s quality of life and offset costs from emergency visits due to suboptimal seizure control.
Closing Remarks
In addition to clinical experience, there is a growing body of literature regarding the pharmacokinetics and clinical use of AACDs in small animal practice. The apparent efficacy and limited adverse effects associated with AACDs make them an attractive treatment option for all common seizure disorders in which long-term administration of an anticonvulsant is warranted.AcknowledgmentThe author would like to thank Terry Lawrence, medical illustrator at the Virginia-Maryland Regional College of Veterinary Medicine, for creating Figures 1 and 2.
AACD = alternative anticonvulsant drug, GABA = γ-aminobutyric acidTable: AMPA = α-amino-3-hydroxy-5 methyl-4-isoxazolepropionic acid receptor (an ionotropic glutamate receptor), GABA = γ-aminobutyric acid, KCS = keratoconjunctivitis sicca, NMDA = N-methyl-d-aspartate receptor (an ionotropic glutamate receptor), SV2A = synaptic vesicle protein 2A, VG = voltage-gated