Acute Pleural Effusion in a Dog
Fenway Chang, BVM, Lakeshore Veterinary Specialists, Glendale, Wisconsin
Andrew Linklater, DVM, DACVECC, Veterinary Specialists of the Rockies, Castle Rock, Colorado
Marcus, a 3-year-old, 77-lb (35-kg) neutered male golden retriever crossbreed, was presented for a 3-day history of increased respiratory effort. The owner noted Marcus had been wheezing when at rest but not coughing or gagging. He was current on vaccinations and flea and tick preventives and had no known trauma, foreign body ingestion, or tick or toxin exposure.
Physical Examination
On examination, Marcus was tachypneic with a respiratory rate of 60 bpm and a mild increase in expiratory effort. His heart rate was 144 bpm, mucous membranes were pink, and pulses were strong and synchronous. His temperature was mildly elevated at 103.2°F (39.6°C). On auscultation, heart and lung sounds were normal on the right side but decreased on the left. The remainder of the examination was unremarkable.
Diagnosis
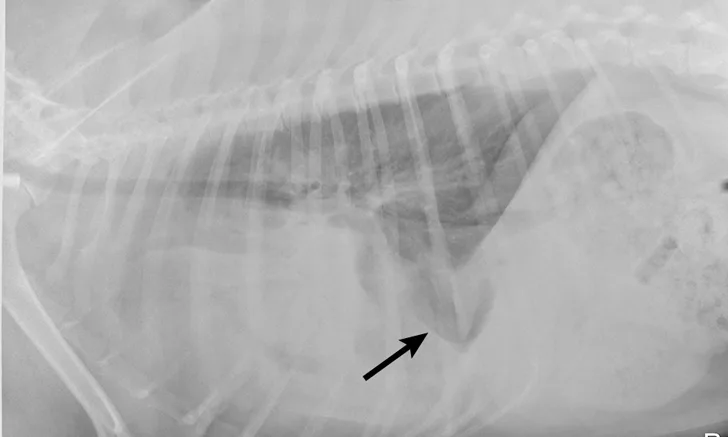
Right lateral thoracic radiographs revealed a large amount of fluid/soft tissue opacity obscuring the cardiac silhouette. Ventrodorsal radiographs showed increased soft tissue opacity in the left hemithorax, primarily in the cranial and middle lung fields. A mild interstitial pattern, a pleural fissure line, and border effacement of the heart were noted in the left hemithorax (Figure 1).
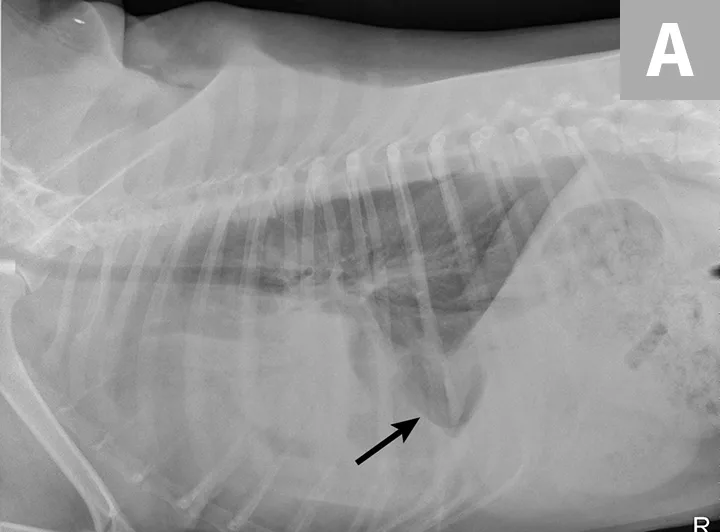
FIGURE 1A
Right lateral (A) and ventrodorsal (B) thoracic radiographs obtained prior to thoracocentesis demonstrating a mild interstitial pattern, moderate pleural effusion, soft tissue opacity in the left cranial and middle lung field, border effacement of the heart, and a pleural fissure line (arrows)
Radiography findings suggested a combination of pulmonary and pleural space disease. Differential diagnoses included pleural effusion (eg, hemothorax, pyothorax, chylothorax, hydrothorax, neoplasia) and pleural space mass or mass effect (eg, neoplasia, lung lobe consolidation or torsion, abscess/granuloma).
CBC, serum chemistry profile, venous blood gas, urinalysis, and coagulation panel (prothrombin time, activated partial thromboplastin time) results were within normal limits.
Pleural effusion was confirmed via thoracic ultrasonography, and therapeutic thoracocentesis was performed; 650 mL of serosanguinous fluid was removed from the left side (Figure 2) and 150 mL from the right side. Packed cell volume and total solids of the fluid were 4% and 3.5 g/dL, respectively, and cytology demonstrated a chronic hemorrhagic, neutrophilic, and histiocytic inflammatory exudate with mesothelial cell hyperplasia, suggesting inflammation without overt evidence of infection or neoplasia.
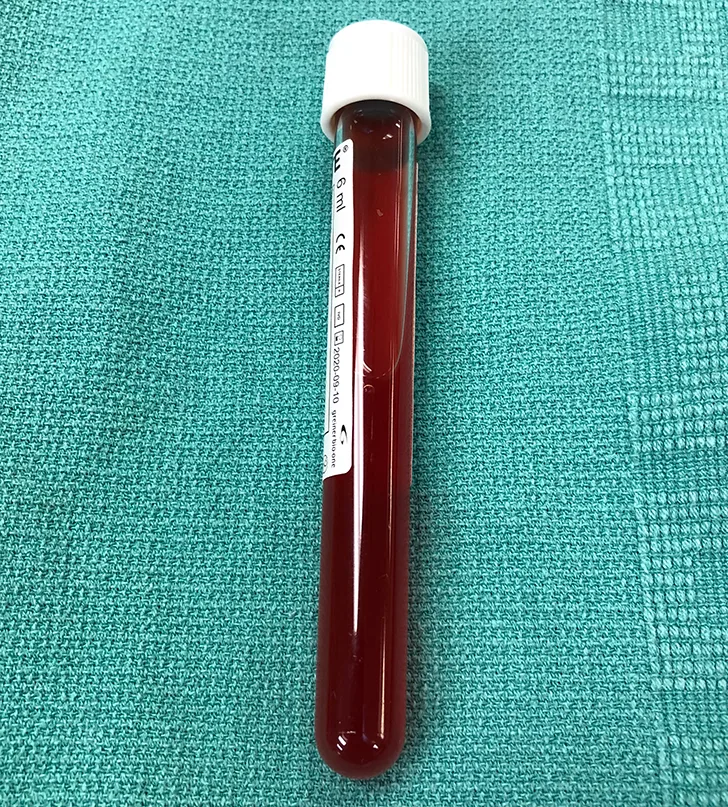
FIGURE 2 A sample of the serosanguinous fluid removed from the thoracic cavity with the initial centesis. This preoperative fluid is substantially different in appearance from the fluid collected postoperatively (Figure 6).
Radiographs obtained after thoracocentesis demonstrated improved pleural effusion and consolidation of the left middle lung lobe (Figure 3). Based on the soft tissue bulge near the hilum and the air bronchocram extending cranially, the primary differential was lung lobe torsion (LLT). Other considerations included pulmonary mass, abscess, or granuloma.
CT demonstrated bilateral moderate pleural effusion with an abnormal appearance of the left cranial lung lobe, stippled gas collections, and truncation of the bronchus that suggested LLT (Figure 4).
Left lateral thoracotomy revealed LLT in the caudal portion of the left cranial lung lobe, and a lung lobectomy was performed. A 20 Fr thoracostomy tube and pleural catheter were placed for postoperative recovery, monitoring, and pain control (Figure 5). Two days postoperation, the amount of pleural effusion aspirated from the thoracostomy tube was still significant in quantity (72 mL/kg/day) and off-white in color (Figure 6). The fluid was confirmed to be chylous effusion based on cytologic examination results (ie, lipid droplets, moderate numbers of mature lymphocytes, and a few macrophages) and paired serum (95 mg/dL) and effusion (819 mg/dL) triglyceride levels.
DIAGNOSIS: LUNG LUBE TORSION
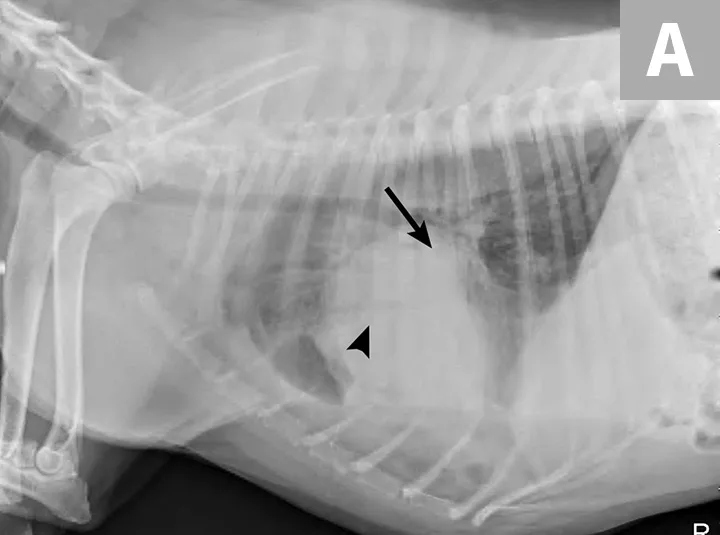
FIGURE 3A
Right lateral (A) and ventrodorsal (B) thoracic radiographs obtained after thoracocentesis showing improvement in pleural effusion with a persistent soft tissue opacity in the left mid-to-cranial thorax. An air bronchogram is seen extending cranially from the hilus (arrowhead) where a subtle bulge (arrow) is present.
Treatment & Management
Fluid continued to be produced in large quantities over the next week, with a plateau at >30 mL/kg/day. Treatment options discussed with the owner included medical management, placement of a long-term SC pleural port, and surgery. Surgery was elected, and pericardiectomy, thoracic duct ligation, and ablation of the cisterna chyli were performed.
TREATMENT AT A GLANCE
Thoracocentesis should be performed when pleural effusion is present.
Chylothorax should be investigated preoperatively if LLT is suspected prior to surgical intervention. Additional surgery at the time of lung lobectomy may be necessary.
LLT is best treated with surgical removal of the affected lobe.
Idiopathic chylothorax should be treated surgically for the best outcome; medical management has limited success.
Refractory chylothorax cases may be managed with placement of a permanent indwelling pleural access catheter.
Prognosis & Outcome
Marcus did well during the second surgical procedure; chylothorax resolved quickly, and he was discharged after 3 days. His condition was reported as normal 3 years after the procedure.
Discussion
LLT can be idiopathic or secondary to pleural effusion, other pulmonary or pleural space disease, trauma, or thoracic surgery.1,2 Deep-chested, large-breed dogs (especially Afghan hounds) and certain small-breed dogs (eg, pugs) have been reported to have a higher occurrence of LLT.1-6 Lobectomy of the affected lung lobe is the treatment of choice for LLT. Previously, survival rates were 50% to 78%1,2,6; however, more recent studies report a survival-to-discharge rate of 92%.4
Chylothorax can occur secondary to intrathoracic pathology that causes obstruction of the thoracic duct and normal lymph flow. Common causes include granuloma, trauma, congenital abnormalities of the thoracic duct, diaphragmatic hernia, cardiac disease, thoracic surgery, and intrathoracic neoplasia.7,8 Although the underlying cause of chylothorax should be treated, a primary cause (ie, idiopathic chylothorax) is not identified in many cases.7
Chylothorax is a common pre- and postoperative finding with LLT.1,2-5,9 Pleural effusion is thought to increase the risk for LLT, and chylothorax that develops after lung lobectomy may be caused by trauma to the thoracic duct during surgery or pleuritis from LLT, which alters lymphatic flow.1,2,9 When LLT is diagnosed and pleural effusion is present, presurgical serum and fluid triglycerides should be tested to diagnose chylous effusion, as chyle may not always have a milky appearance, and the presence of chylothorax may warrant additional surgical procedures at the time of lung lobectomy.5
Several therapeutic options are available for idiopathic chylothorax.10-16 Medical options include feeding a low-fat, medium-chain triglyceride diet and administering rutin (a benzypyrone) with or without octreotide (a somatostatin analog). Medical therapy alone has a low success rate (eg, 40%).10-12 Surgical options include ligation of the thoracic duct, subtotal pericardiectomy, and cisterna chyli ablation. Success rates of 53% to 88% have been reported when these surgical procedures are used in combination.10,13-16 Other surgical procedures with variable success rates have also been reported, including thoracic omentalization, pleurodesis, placement of pleuroperitoneal or pleurovenous shunts, and placement of permanent pleural space catheters for intermittent evacuation of fluid.10,13-16
Take-Home Messages
LLT should be suspected in young patients presented with acute pleural effusion and possible lung consolidation but are otherwise healthy. Certain breeds may be predisposed to LLT.
LLT is best diagnosed with CT or surgery. The index of suspicion is raised when focal soft tissue density in a lung lobe is seen on routine radiographs with or without pleural effusion. A stippled appearance of the lung field, indicating trapped air or truncated bronchus, may also be present.17
When LLT is diagnosed and pleural effusion is present, serum and fluid triglycerides should be compared preoperatively to determine the presence of chyle.
Chylothorax is diagnosed when fluid triglycerides are ≥2 times higher than serum triglycerides in a fasted patient. Cytologic findings can help confirm the diagnosis.
If the underlying cause of chylothorax is undetermined or untreatable, medical management may be attempted but typically has limited success. Surgical options have improved, but outcomes are variable.