Acute Pancreatitis in Dogs
Daniel K. Langlois, DVM, DACVIM (SAIM), Michigan State University
Harry Cridge, MVB, MS, DACVIM (SAIM), MRCVS, Michigan State University

Pancreatitis is the most common disorder of the exocrine pancreas in dogs.1 Although exact incidence rates are unknown, pancreatic inflammation is commonly observed in necropsy samples.2 Acute pancreatitis is characterized histopathologically by neutrophilic inflammation, edema, and necrosis3; however, histopathology is seldom performed in clinical settings, and diagnosis is often based on clinical and laboratory features of disease (see Diagnosis).
The exact pathophysiology of acute pancreatitis is not completely understood, but a commonly described pathophysiologic mechanism is inappropriate fusion of zymogen and lysosomal granules in pancreatic acinar cells that leads to premature activation of trypsinogen.4,5 Oxidative stress and hypotension are potential triggers; additional factors may also contribute. A complex inflammatory cascade and cytokine storm occur after activated digestive enzymes overwhelm normal cellular defense mechanisms,4,5 resulting in local and systemic consequences.
History & Clinical Signs
Dogs without any identifiable risk factors can develop acute pancreatitis, but it is often reported in middle-aged and older dogs.6 Certain breeds (eg, miniature schnauzers, Yorkshire terriers, other terrier breeds) are overrepresented.6-8 Dietary indiscretion and ingestion of table scraps are known risk factors.9 Hyperlipidemia and diseases associated with hyperlipidemia (eg, diabetes mellitus, hyperadrenocorticism, hypothyroidism) may predispose dogs to acute pancreatitis.6,10-14 Obesity, recent anesthetic events, dietary fat content, sex, neuter status, and certain medications (eg, anticonvulsants) are also suggested risk factors.6,9
Patients with acute pancreatitis typically have acute or peracute vomiting, dehydration, and anorexia often accompanied by abdominal pain.1,7,8 Weakness, fever, diarrhea, polyuria/polydipsia, neurologic deficits, and GI hemorrhage are variably reported.1,4,7,8
Increased disease awareness has led to recognition of atypical clinical signs. Dogs with mild disease may be presented with dysrexia alone, whereas dogs with severe disease may also have cardiovascular shock or disseminated intravascular coagulation.8 Other potential findings include abdominal effusion, icterus due to posthepatic biliary obstruction, and respiratory distress associated with aspiration pneumonia or severe systemic inflammation.11,15-17
Diagnosis
Histopathology has limited use when diagnosing pancreatitis due to invasiveness, risk for missing localized lesions, and potential for false-positive results due to subclinical inflammation.18,19 Integration of clinical features, routine laboratory evaluations, diagnostic imaging, and serum pancreatic lipase measurement are recommended for presumptive diagnosis. Diagnostic imaging and pancreas-specific lipase measurements are indicated if clinical signs and laboratory evaluations are consistent with acute pancreatitis.1,4
Laboratory Evaluations
Hematologic and routine serum chemistry evaluations can help exclude other causes of illness and assess consequences of pancreatitis. Laboratory abnormalities consistent with dehydration, vomiting, and generalized inflammation are variably reported.1,7 Regional inflammation, portal drainage of the pancreas, and cholestasis commonly result in mild to severe increases in liver enzyme activities, with ALP activity usually greater than ALT activity.7 Azotemia due to acute kidney injury and hyperbilirubinemia due to bile duct obstruction can occur in severe cases.1,7,16,20 Anecdotally, dogs with severe disease often have more extensive hematologic and serum chemistry abnormalities than dogs with mild disease, in which there may be minimal or no abnormalities. Severity of certain laboratory abnormalities is incorporated into validated scoring schematics, which may help predict outcomes.21
Although amylase and lipase are often included in routine serum chemistry profiles, their diagnostic value is questionable because sensitivity for disease detection is poor, and variable specificity has been reported.22,23
Diagnostic Imaging
Abdominal radiography can help rule out some acute abdominal diseases (eg, mechanical obstruction) but are of limited value for diagnosing acute pancreatitis.7
Acute pancreatitis is most frequently evaluated via abdominal ultrasonography (Figure).24-26 Findings suggestive of acute pancreatitis include pancreatic enlargement, hypoechogenicity or altered echogenicity of pancreatic parenchyma, hyperechogenicity of surrounding mesentery, and dilation of pancreatic or biliary ducts. Other nonspecific findings include ascites, gastric wall thickening, lateral displacement of the duodenum, and intestinal ileus.24-26 Discordant sensitivity and specificity of ultrasound for diagnosis of acute pancreatitis have been reported; variable results are influenced by differing case definitions for acute pancreatitis and number of ultrasound criteria used for diagnosis.7,27 Ultrasound use is also presumably related to other factors (eg, equipment quality, clinician expertise). Results from one study suggested high sensitivity and poor specificity when only 1 or 2 ultrasonographic features were required for acute pancreatitis diagnosis; poor sensitivity and high specificity were found when ≥3 features were required for diagnosis.27
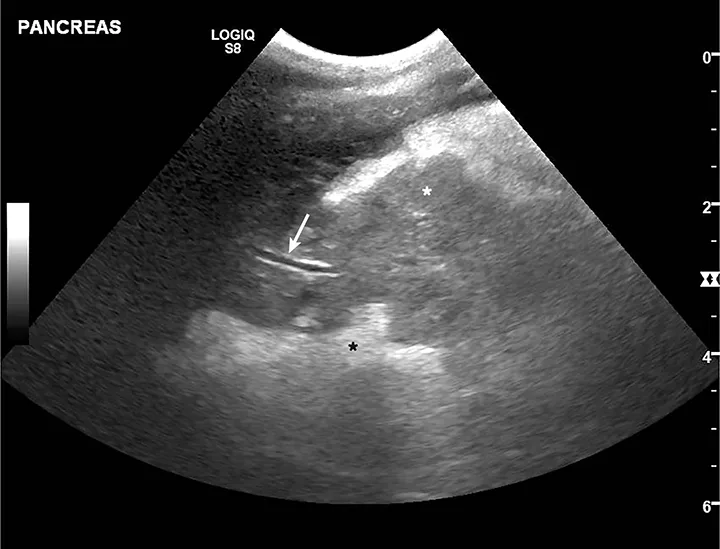
Ultrasound image of a 10-year-old spayed fox terrier with classic acute pancreatitis findings. Pancreas is enlarged and diffusely hypoechoic (white star; pancreatic mesentery) with undulating margins. Surrounding mesentery is diffusely hyperechoic (black star; peripancreatic fat). Normal pancreatic vein (arrow) can be seen.
Pancreas-Specific Lipase Measurements & Other Assays
Serum pancreas-specific lipase concentrations increase during acute pancreatitis, and pancreas-specific lipase may be one of the most accurate blood-based tests that can be used to diagnose acute pancreatitis in dogs.28,29 Normal results on a semiquantitative patient-side pancreas-specific lipase assay can be useful in ruling out acute pancreatitis.30 False-positives can occur; therefore, a positive result alone should not be used for definitive diagnosis.31 A quantitative pancreas-specific lipase assay from a reference laboratory may have more value in confirming diagnosis.1 Similar to ultrasonography, reported sensitivity and specificity of quantitative pancreas-specific lipase are variable as a result of differing study designs and case definitions.28,29,31 If a clinical reference standard is used to define acute pancreatitis cases, reported sensitivity and specificity of quantitative pancreas-specific lipase range from 70% to 91% and 74% to 88%, respectively.32 Despite this wide range, quantitative pancreas-specific lipase assays still appear to have the best performance among common blood-based biomarkers for acute pancreatitis.29
Several newer assays measure lipase activity based on breakdown of substrate 1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6’-methylresorufin) ester (ie, DGGR hydrolysis), which is speculated to be more selective than lipase measurements included on routine serum chemistry profiles. Although DGGR-based assays have good correlation with other reference laboratory quantitative assays, results may be affected by nonpancreatic lipase, and additional studies are needed to determine clinical significance.33 Other assays are available, but clinical and analytic performance data should be evaluated prior to routine use.
Confirming Diagnosis
Acute pancreatitis can be diagnosed when consistent clinical signs are present, reference laboratory quantitative pancreas-specific lipase concentrations are >400 µg/L (normal, ≤200 µg/L), and ≥2 ultrasound findings are consistent with diagnosis of acute pancreatitis.27-29,31,32 Conversely, acute pancreatitis is unlikely when pancreas-specific lipase concentrations are <400 µg/L and ultrasound findings are normal. Equivocal and conflicting results should be interpreted based on the clinical picture; repeat testing may be required.34
Treatment
Dogs with acute pancreatitis usually require several days of hospitalization and supportive care. Recommendations continually evolve, but IV fluids, antiemetics, pain control, and nutritional support are mainstays of treatment.4,35
IV fluid therapy with isotonic crystalloids (eg, lactated Ringer’s solution) corrects dehydration and hypovolemia and promotes normal pancreatic blood flow.36 Antiemetic medications are recommended, including maropitant (1 mg/kg IV every 24 hours) for vomiting and ondansetron (0.5 mg/kg slow IV every 8-12 hours) for nausea. Opioid analgesics (eg, methadone, 0.1-0.4 mg/kg IV, IM, or SC every 4-8 hours; fentanyl, 3-5 µg/kg/hour CRI; buprenorphine, 0.005-0.03 mg/kg IV, IM, or SC every 6-12 hours) are often required for pain control, but it is important to consider the clinical status of the patient when selecting specific drugs and dosages. NSAIDs are not recommended in dogs with acute GI disease, including those with acute pancreatitis.35,37 Ketamine (0.3-0.6 mg/kg/hour CRI) or lidocaine (1.5-3.0 mg/kg/hour CRI) infusions in conjunction with fentanyl can help patients with severe or refractory pain. Pain control should be regularly monitored with a validated scoring system (eg, Glasgow Composite Measure Pain Scale).38
Acid suppressants (eg, pantoprazole, 1 mg/kg IV every 12 hours; omeprazole, 1 mg/kg PO every 12 hours) were traditionally used in acute pancreatitis treatment, but studies have not been conducted to determine whether they are beneficial or detrimental. There is currently no evidence indicating acid suppressants are needed unless a patient has concurrent gastric ulceration or erosion.39
Dogs with mild acute pancreatitis may resume eating soon after treatment is initiated, but those with moderate to severe disease tend to remain dysrexic. Enteral nutritional support is generally recommended in these cases.40 Mechanistic studies and extrapolations from other species suggest early enteral nutrition can improve outcome.41 Studies in veterinary medicine are limited, and the appropriate time to initiate feeding is not fully known,35,40,42 but prolonged fasting to decrease pancreatic stimulation is no longer recommended.
Some clinicians initiate enteral nutritional support 1 to 2 days after hospitalization to allow time for correction of fluid deficits and electrolyte abnormalities and to appropriately control pain and nausea. Others initiate nutritional support as soon as possible. Duration of dysrexia preceding hospitalization should be considered. Nutritional support (ideally with a fat-restricted diet) is most frequently administered via nasoesophageal, nasogastric, or esophageal feeding tube. Small-volume, intermittent feedings or slow, continuous infusions (15%-25% resting energy requirements on the first day) are recommended to avoid feeding-associated complications (eg, vomiting, abdominal pain). In humans, meeting 100% of caloric requirements does not appear to be necessary for improved outcome; this supports starting with small-volume feeding and gradual escalation.43
Controversial treatments (ie, metoclopramide, antibiotics, glucocorticoids, plasma transfusions) are summarized in Table 1.4,15,35,44,45 Routine use of these treatments in all patients is not recommended, although they can be useful in select cases.
Dogs should remain hospitalized until hydration status has normalized, pain and nausea are controlled, and voluntary food intake has returned. A low-fat diet is typically prescribed for at least 2 to 4 weeks, although the length of time a fat-restricted diet should be fed is unknown; a lifelong fat-restricted diet is sometimes preferred.
Table 1: Controversial Treatments for Acute Pancreatitis in Dogs
Prognosis
Mortality has been reported from <20% to >50%; this range likely reflects disease severity.21,46,47
Canine acute pancreatitis severity score can help predict outcome (Table 2).21 A score >11 was shown to have 86% sensitivity and 92% specificity for predicting short-term mortality rates in dogs with acute pancreatitis.21
Although scoring systems are most useful in research settings, they highlight abnormalities associated with worse prognosis. Variables, including ascites, respiratory distress, renal azotemia, hyponatremia, ionized hypocalcemia, increased C-reactive protein, and decreased antithrombin activity, have been associated with increased risk for mortality.4,17,21,46-49
Dogs with mild disease generally have a favorable prognosis. Dogs with severe acute pancreatitis and systemic complications are more likely to succumb to disease.
Table 2: Canine Acute Pancreatitis Severity Score
*A coagulation disorder must have thrombocytopenia (<63,000/µL) or prothrombin time or activated partial thromboplastin time >25% above the reference interval.21
†Systemic inflammatory response syndrome must have ≥2 of the following: temperature <100.6°F (38°C) or >102.6°F (39°C), heart rate >120 beats per minute, respiratory rate >20 breaths per minute, WBC count <6,000/µL or >16,000/µL, or band neutrophils >3% of the total WBC count.21
Clinical Follow-Up & Prevention
Follow-up examinations and routine laboratory evaluations (ie, CBC, serum chemistry profile with triglyceride measurement) should be performed 1 to 2 weeks after discharge. Serum chemistry abnormalities, especially hyperlipidemia, should prompt further diagnostic investigation and treatment.13,14 Dogs with normal serum chemistry profile results without additional risk factors for acute pancreatitis may be gradually transitioned to their original life-stage–appropriate diet, with a clinical assumption that acute pancreatitis may have been an isolated event. A long-term low-fat diet is recommended in dogs with recurrent acute pancreatitis and in dogs with hyperlipidemia or other active risk factors.
In the authors’ experience, initial and recurrent acute pancreatitis appear to be more common in dogs with poorly managed comorbidities (eg, diabetes mellitus). Avoiding risk factors may help prevent recurrence.