Acute Onset of Tetraparesis in a Dog
Mark Troxel, DVM, DACVIM (Neurology), Massachusetts Veterinary Referral Hospital, Woburn, Massachusetts

A 6-year-old spayed German shorthaired pointer presented for neurologic evaluation of tetraparesis.
History
Approximately 4 days before presentation, the dog was suddenly ataxic in all limbs and showed reluctance to use the stairs. The condition improved somewhat over 2 days, but soon clinical signs rapidly progressed; the patient became unable to walk and was presented to the specialty neurology practice. Immediately after admission, the dog experienced a generalized tonic–clonic seizure. There was neither recent trauma nor travel history during the past year.
Related Article: Canine Idiopathic Inflammatory CNS Disease
Examination Findings
Other than neurologic abnormalities, physical examination findings were within normal limits. The dog was mentally dull and nonambulatory tetraparetic. When encouraged to walk with support, the dog was very ataxic with a wide-stanced gait on all limbs and a tendency to veer and fall toward its left. Truncal sway was present when standing still.
Cranial nerve examination was within normal limits. Conscious proprioception and hopping were absent in all limbs. Myotatic and withdrawal reflexes were within normal limits in all limbs. Paraspinal hyperesthesia was noted on neck palpation and during range-of-motion evaluation of the head and neck.
Related Article: Progressive Behavioral Changes in a Dog
Ask Yourself...
1. What is the neuroanatomic localization for this patient’s clinical signs?2. What diagnostic differentials should be considered?3. What diagnostic tests should be recommended in this case?
Diagnosis
Multifocal CNS disease
Preliminary Diagnosis
Given the patient’s signalment and history, the most common diagnostic differentials would be noninfectious immune-mediated inflammatory meningoencephalomyelitis, infectious meningoencephalomyelitis, and intracranial neoplasia. Other possibilities (Table: Diagnostic Differentials for Adult Dogs with Multifocal CNS Signs) are less common.
Diagnostic Measures
The patient was admitted to the hospital for an MRI, cerebrospinal fluid (CSF) analysis, and testing for possible infectious disease. Results of preanesthetic CBC, chemistry panel, total thyroxine (TT4), and urinalysis were unremarkable. The MRI (Figure 1) revealed lesions throughout the brain, most prominently in the cerebellum, brainstem, and cranial cervical spinal cord. Multifocal T2-weighted hyperintensities of the cerebellum, medulla oblongata, and cranial cervical spinal cord were noted. These regions were generally hyperintense on fluid-attenuated inversion recovery (FLAIR) images and isointense on T1-weighted images and displayed moderate, patchy contrast enhancement. Excessive contrast enhancement of the meninges around the brainstem and cerebral hemispheres was also noted. In addition, MRI also showed C2–3 intervertebral disk protrusion with mild-to-moderate spinal cord compression, which was considered an incidental finding.
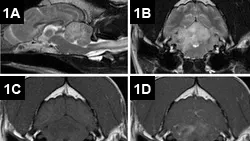
Figure 1. MRI of the brain: Sagittal T2-weighted images reveal multifocal, hyperintense intraaxial lesions of the brainstem, cerebellum, and cranial cervical spinal cord (A). Axial T2-weighted images at the level of the cerebellum showed marked hyperintensity of the cerebellum and right lateral medulla oblongata (B). The lesions were isointense to hypo-intense on precontrast T1-weighted images (C) and displayed moderate, patchy contrast enhancement (D).
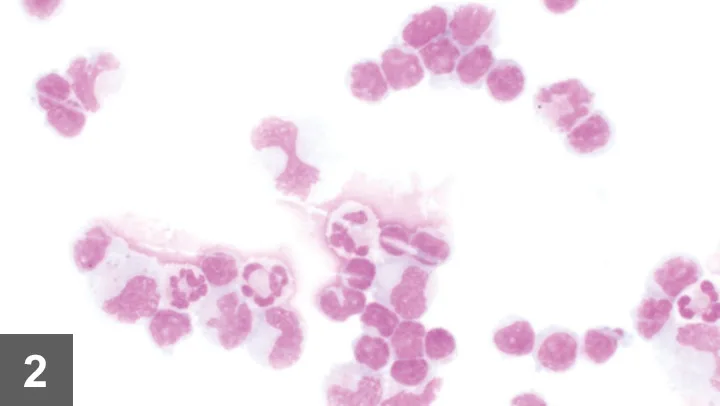
The CSF appeared colorless and hazy. Protein level (718 mg/dL; normal, <25 mg/dL) and leukocyte count (802 cells/μL; normal, <5 cells/mL) were markedly elevated. Differential cytology (Figure 2) showed marked mixed-cell pleocytosis (44% nondegenerate neutrophils, 41% small lymphocytes, 15% large mononuclear cells).
Figure 2. CSF cytology showed marked mixed-cell pleocytosis with neutrophils, large mononuclear cells (macrophages), and small lymphocytes.
CSF aerobic culture and infectious disease titers were submitted to a reference laboratory. Infectious diseases can cause similar MRI and CSF alterations; however, because of the severity of clinical signs and increased likelihood of immune-mediated disease (eg, granulomatous meningoencephalomyelitis [GME]), the patient was started on an immunosuppressive dose of prednisone at 1 mg/kg PO q12h, clindamycin at 10 mg/kg PO q12h, and doxycycline at 5 mg/kg PO q12h and provided the general supportive care for a nonambulatory dog.
CSF aerobic culture and sensitivity results were negative. The dog was also negative for canine distemper virus and Cryptococcus neoformans on CSF and for Toxoplasma gondii, Neospora caninum, Borrelia burgdorferi, Anaplasma phagocytophilum, Ehrlichia cani_s, and _Rickettsia rickettsii on serum analysis. Tick-borne diseases, especially associated with A phagocytophilum, E canis, and R rickettsia infection, have been implicated in causing CNS disease.
Did You Answer?
1. The neurologic examination was consistent with a multifocal CNS disease process. The generalized tonic–clonic seizure indicated forebrain dysfunction. The predominant signs of tetraparesis, vestibular ataxia, truncal sway, and postural reaction deficits were suggestive of cerebellar and central vestibular (brainstem) dysfunction. Cervical pain was suggestive of cervical spinal cord disease or meningeal involvement; however, some patients with intracranial disease have referred cervical pain.
2. In an older dog with progressive signs referable to the CNS, common causes include inflammatory disease (eg, meningoencephalomyelitis, neoplasia). Additional differentials should be considered (Table above: Diagnostic Differentials for Adult Dogs with Multifocal CNS Signs).
3. Minimum database includes a CBC, chemistry panel, TT4 level, and urinalysis. TT4 should be evaluated in geriatric patients, because hypothyroidism can cause similar clinical signs and exacerbate weakness. Because of the generalized tonic-clonic seizure that occurred and in case phenobarbital would be required later, TT4 testing was completed to obtain a baseline, as phenobarbital lowers these levels.
Figure 3A. Pathology samples from another dog with similar histopathologic findings: Multifocal areas of malacia (brown–gray spots) in the brainstem (most prominent to the right of midline) and cerebellar white matter are visible on gross pathology samples.
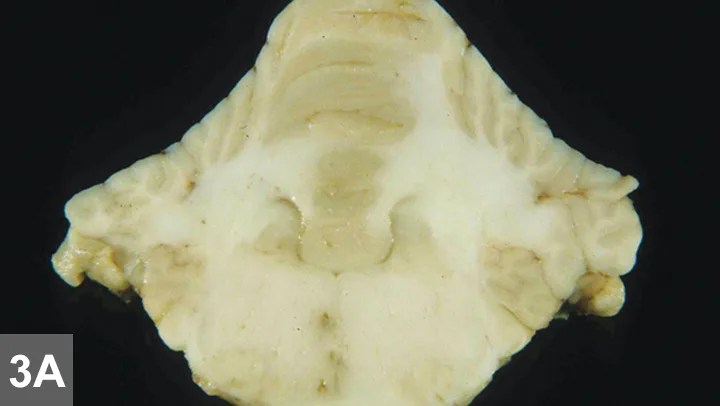
Figure 3B. Low-power histologic examination showed areas of intraparenchymal perivascular cuffing and area of inflammation in the parenchyma (arrows).
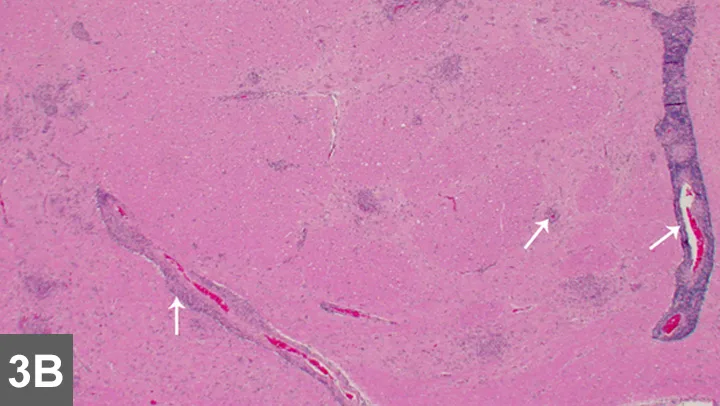
Figure 3C. High-power examination of the brain demonstrated a mixed inflammatory cell perivascular cuffing. Courtesy of Dr. Tom Van Winkle
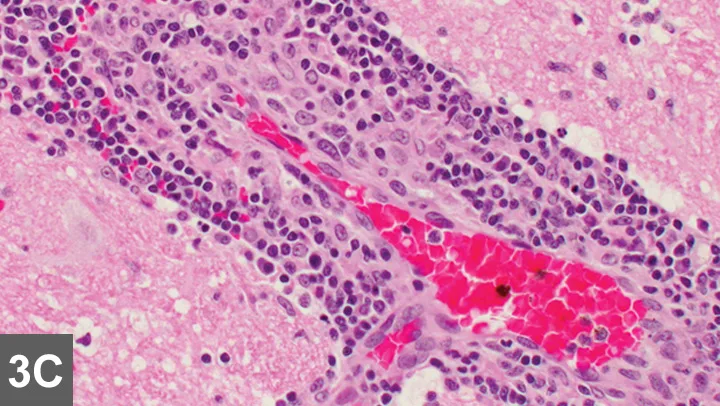
In older patients, thoracic radiography is recommended as a preanesthetic screening tool before MRI to rule out metastatic or primary neoplasia, cardiovascular disease, and pneumonia, which might preclude advanced imaging. MRI and CSF analysis are the hallmarks for diagnosis of intracranial disease. Infectious disease titers are often performed to rule out infectious inflammatory disease. Titers performed should be based on regional exposure risk. Histopathology (Figure 3) remains standard for diagnosing GME and other noninfectious, immune-mediated inflammatory CNS diseases. Stereotactic or surgical biopsy has been described but not commonly performed, and the diagnosis usually remains presumptive.
Diagnosis
Based on MRI and CSF results, combined with negative infectious disease testing, the presumptive diagnosis was GME, although brain biopsy samples were not collected to confirm this diagnosis. Neoplasia (eg, lymphoma, gliomatosis cerebri), other forms of noninfectious and immune-mediated encephalitis (eg, necrotizing leukoencephalitis, necrotizing meningoencephalitis), and other infectious encephalitides could not be entirely excluded. Although the terminology is controversial, some veterinary neurologists use meningoencephalitis of unknown etiology when histopathology has not been performed.
Treatment
Because the patient showed no improvement for the first 2 days, a single dose of lomustine at 60 mg/m2 was administered as an additional immunosuppressive agent. Prednisone, clindamycin, and doxycycline were continued at the same doses. The dog developed a soft, productive cough, but thoracic radiographs were unremarkable. Enrofloxacin at 10 mg/kg PO q24h was started empirically for possible early aspiration pneumonia in light of treatment with immunosuppressive drugs. Although the patient was still nonambulatory, it was discharged at the owners’ request on day 5. Cytarabine injections were recommended, but the owners elected to initiate cyclosporine at 3 mg/kg PO q12h, owing to schedule restrictions and inability to transport the dog for in-hospital treatment and monitoring.
The dog was discharged on prednisone, clindamycin, doxycycline, and enrofloxacin at the same doses. Over the following week, the dog slowly regained ability to walk, albeit with moderate cerebellar–vestibular ataxia. To allow quicker tapering of prednisone, thereby avoiding adverse effects (eg, polyuria/polydipsia) of prednisone and its associated changes to owner quality of life, the owners agreed to start cytarabine injections (cytosine arabinoside, 50 mg/m2 SC q12h for 2 days). Two weeks later, the dog’s neurologic status was significantly improved with only mild ataxia. The cytosine arabinoside was continued at the same dose q3wk for 4 rounds of treatment, and the prednisone dose was concurrently lowered by approximately 25% q3–4wk.
The dog achieved full recovery in 2 months and remained clinically normal as dosages were tapered. After 4 rounds of injections, the cytarabine treatments were decreased to q4wk for 4 rounds of treatment and then q6wk for 4 rounds of treatment. At the owners’ request (ie, financial reasons, patient appeared clinically normal), cyclosporine was discontinued after 2 months. Cytarabine was discontinued 10 months later, and the dog remained clinically normal on low-dose prednisone (0.25 mg/kg PO q48h) for 2 years based on serial neurologic examinations. To reduce risk for recurrence, the owners elected long-term prednisone rather than weaning the dog off medication entirely.