Acute Onset of Skin Lesions in a Dog
An 8-year-old, 8.2-kg spayed female terrier mix was presented for the acute onset of skin lesions.
History. The skin lesions were not pruritic and had been present for 2 weeks. Fourteen months previously, the dog had been diagnosed with immune-mediated polyarthritis (IMPA); it had been well controlled for more than 6 months with prednisone (0.3 mg/kg Q 48 H).
One year ago, the dog had an episode of pancreatitis. In addition to prednisone, the dog had been receiving famotidine (5 mg Q 24 H) and ivermectin (68 mcg monthly). Vaccinations were last administered 18 months ago. The dog had known tick and foxtail exposure.
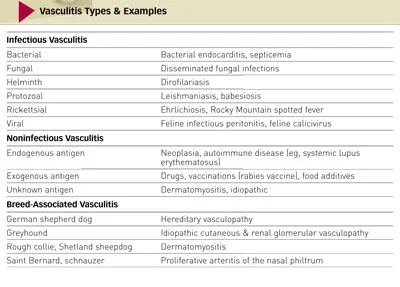
Physical Examination. The dog was alert, responsive, and hydrated. Mild temporal muscle atrophy was present. Dermatologic examination revealed multifocal, erythematous, circular to targetoid macular lesions over the ventral abdomen; visible comedones and mild scaling were also present (Figure 1). Some lesions appeared more hemorrhagic (Figure 2). There were no lesions within the oral cavity. All 4 feet had paw pads with discrete, circular central lesions that varied from slightly depressed lesions of pallor (Figure 3) to hemorrhagic crusts with concentric healing (Figure 4).
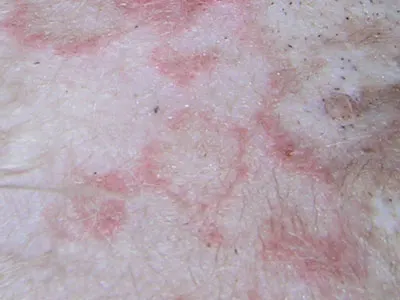
Figure 1: Erythematous circular and targetlike macules
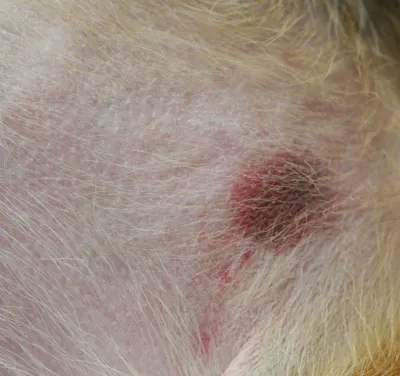
Figure 2: Hemorrhagic lesion

Figure 3: Foot pad lesions of central pallor, which are slightly depressed

Figure 4: More severe foot pad lesions with central hemorrhagic crusts andconcentric healing lesions
Laboratory Diagnostics. The lesions on the ventral abdomen did not blanch on diascopy (Figure 5). A CBC revealed normal cell counts but a mild elevation in band neutrophils. Serum alkaline phosphatase and alanine transferase concentrations were also slightly increased. Urinalysis showed no abnormalities.
Cytologic evaluation of acetate tape preparations from lesional skin was unremarkable. Three 6-mm punch biopsy specimens of the haired skin over the ventrum revealed moderate to severe, deep, and superficial neutrophilic vasculitis. Titers for tick-borne rickettsial diseases were negative. Arthrocentesis showed normal to marginal neutrophilic inflammation.
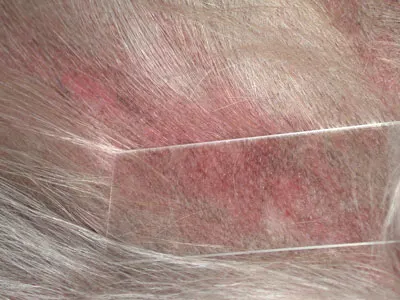
Figure 5: Diascopy with no evidence of blanching
Ask Yourself...
• What are the differential diagnoses for these skin lesions?• How is diascopy performed, and what information can be obtained from this procedure?• Skin biopsy specimens should be obtained from which anatomic sites?• On the basis of these histopathology results, what etiologies should be considered for a patient with this histologic diagnosis?
Did You Answer...
• Differential diagnoses to consider include vasculitis, petechiations from coagulation disturbances, erythema multiforme, cutaneous drug reactions, systemic lupus erythematosus, epitheliotropic T-cell lymphoma. Other less common differentials might include pemphigus foliaceus (if lesions limited to the foot pads), lupoid dermatosis (if dog predisposed breed, which it is not), and leishmaniasis (if recent history of travel to an endemic area).• Diascopy is a test for blanchability performed by applying pressure with a glass slide to observe color changes. The test is performed to determine if an erythematous lesion is the result of inflammation or hemorrhage in the skin. If a lesion blanches, there is vascular dilatation; if it does not, there is hemorrhage in the skin.• Early hemorrhagic lesions are preferred for biopsy over chronic necrotic or ulcerated lesions. Larger punch biopsies or wedge biopsy are better choices to obtain a diagnostic sample. Multiple lesions should be biopsied because diagnostic lesions of vasculitis may not be evident in all sections. Biopsies should be sufficiently deep to ensure that dermal vasculature is sampled. If possible, samples should be taken from the most practical areas to biopsy. Biopsies of the pads were not taken in this case because there were lesions on the ventral abdomen that could be more readily biopsied using only local anesthetic. If foot pad lesions are biopsied, general anesthetic is typically required. Consideration of patient comfort should be taken into account when selecting foot pad lesions to biopsy, and, if possible, biopsies should be limited to 1 foot. Either deep punch biopsies or wedge biopsies can be obtained; wedge biopsies often provide deeper samples but can cause more morbidity than punch biopsies.• Search for an underlying cause after vasculitis has been confirmed histologically. Primary immunologic disease, infectious disease, drug reactions, rabies vaccine, or neoplasia should all be considered as possible underlying etiologies. Rabies vaccine–induced vasculopathy classically targets pinnal margins and tail tips but can become more generalized. Breed-associated vasculitides are also recognized. If an underlying antigenic drive cannot be identified, then the vasculitis is considered idiopathic and should be described according to pathologic evaluation of vessel type, size, location, and inflammatory infiltrate.
DiagnosisCutaneous Vasculitis
Preliminary Diagnosis. The cutaneous lesions were typical for vasculitis, which was confirmed histologically. Vasculitis may involve only 1 organ system (such as the skin) or multiple organ systems with variable clinical signs. Occasionally a primary immunologic disease, vasculitis is more commonly secondary to some other process, such as an infectious disease, drug reaction, reaction to rabies vaccine, or neoplasia (Table).
There was no evidence of underlying infectious disease in this case. Drug-induced vasculitis was possible, and famotidine has been reported to cause leukocytoclastic vasculitis in humans. The dog also had a history of IMPA; therefore, the cutaneous vasculitis was perhaps a progression of a primarysystemic autoimmune disease, such as systemic lupus erythematosus.
Treatment. Famotidine and ivermectin were discontinued in case either was inciting a drug reaction. The dose of prednisone was increased to 0.9 mg/kg Q 12 H, and pentoxifylline was prescribed at a dose of 24 mg/kg Q 8 H. Pentoxifylline has both immunomodulatory and positive rheologic effects, resulting in reduced inflammation and improved blood flow.
Outcome. At a 3-week follow-up appointment, the lesions were 70% improved. The prednisone dose was tapered to 0.6 mg/kg Q 12 H; the pentoxifylline dose was unchanged. Six weeks after initial presentation, all lesions had resolved and the prednisone dose was decreased to 0.6 mg/kg Q 24 H; the pentoxifylline dose was again continued unchanged.
Three months after initial presentation, the dog was presented due to recurring signs of IMPA (prednisone dose, 0.3 mg/kg Q 48 H). On examination, no recurrence of the skin lesions was evident. Cyclosporine (3.1 mg/kg) was added to prednisone to manage the IMPA, and pentoxifylline was continued at the prescribed dose.